# The Dielectric Constant of Water
[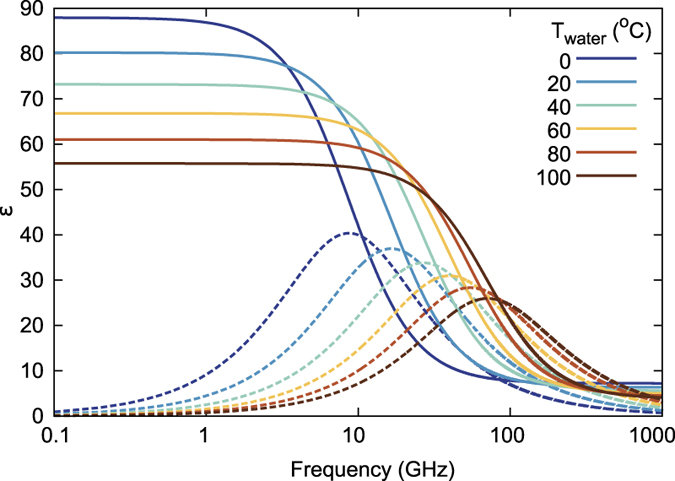](https://stanslegacy.com/uploads/images/gallery/2023-02/OqmJ0MOBPLOrvECj-dielectric-permittivity-of-water-as-a-function-of-frequency-for-the-temperature-0-100c.jpeg)The **dielectric constant**, also known as the **relative permittivity**, is a measure of a material's ability to store electrical energy in an electric field. In the case of water, the dielectric constant is relatively high, making it an effective dielectric material for use in electrical circuits.
When water is used as a dielectric material in an electrical circuit, it behaves as a resistor, limiting the flow of electrical current through the circuit. The resistance of the water depends on several factors, including the concentration of ions and impurities in the water, the temperature, and the applied electric field.
The resistance of water as a dielectric material can be modeled using Ohm's Law, which states that the current flowing through a resistor is directly proportional to the voltage across the resistor and inversely proportional to the resistance of the resistor. In the case of water, the resistance can be affected by the dielectric constant, which determines the amount of electrical energy that can be stored in the water.
The dielectric constant of water is influenced by the presence of ions and molecules in the water, as well as the temperature and pressure of the water. At room temperature and standard pressure, the dielectric constant of water is approximately 80, making it an effective dielectric material for use in electrical circuits.
The dielectric constant of water also influences the capacitance of the circuit, which is a measure of the amount of electrical charge that can be stored in the circuit for a given voltage. The capacitance of a circuit containing water as a dielectric material can be calculated using the equation C = εA/d, where C is the capacitance, ε is the dielectric constant of water, A is the area of the plates in the capacitor, and d is the distance between the plates.
In addition to its use as a dielectric material, water can also act as a capacitor itself, storing electrical charge in the form of ions and molecules in the water. This can lead to self-discharge and leakage currents in the circuit, which can affect the performance of the circuit over time.
| [](https://stanslegacy.com/uploads/images/gallery/2023-02/biwBnl43v5tAoNFU-dielectric-permittivity-spectrum-over-a-wide-range-of-frequencies-various-processes.png) | [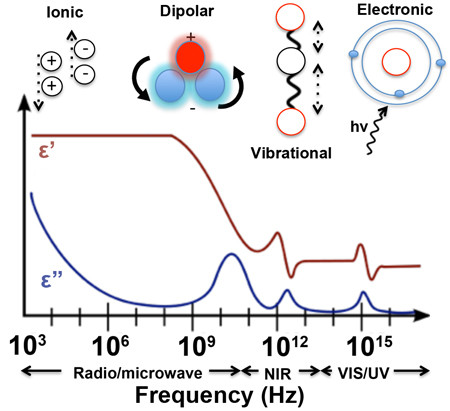](https://stanslegacy.com/uploads/images/gallery/2023-02/JGU1H60yb4LSlyvu-figure-1.jpg) |
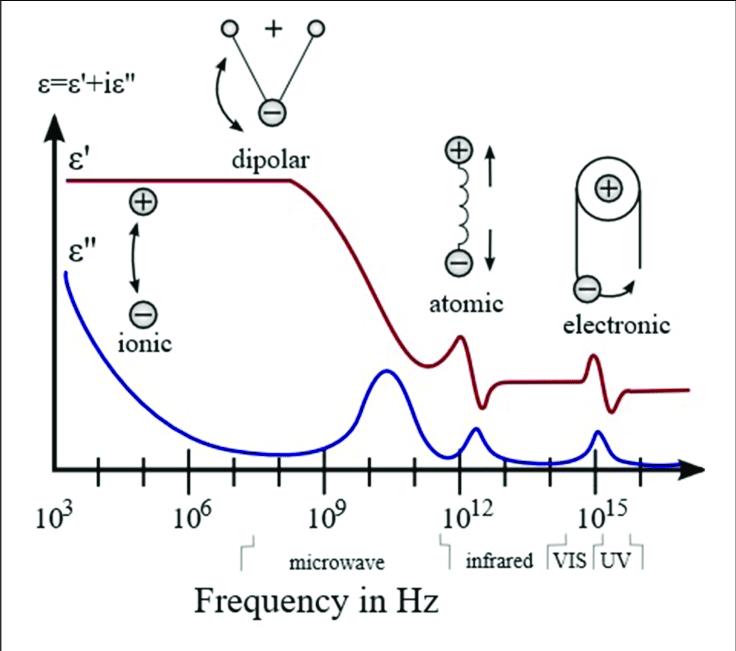
The relative permittivity, also known as the dielectric constant, of water in a capacitor is an important property that affects the behavior of the capacitor. The dielectric constant of water varies with frequency and temperature, and different frequency ranges can have different effects on the various types of polarizability in water.
**Ionic polarizability** in water occurs when ions in the water are attracted to the opposite charge on the plates of the capacitor, causing the water to become polarized. At low frequencies, ionic polarizability dominates, and the dielectric constant of water increases. This can cause the capacitance of the capacitor to increase as well, allowing it to store more charge at low frequencies.
**Atomic** and **vibrational** polarizability in water occurs when the electric field of the capacitor causes the atoms and molecules in the water to vibrate, leading to polarization of the water molecules. This type of polarizability is most effective at intermediate frequencies, typically between 1 MHz and 1 GHz. At these frequencies, the dielectric constant of water is high, and the water can store a significant amount of electrical charge.
**Electric** polarizability in water occurs when the water molecules become aligned in response to the electric field of the capacitor. This type of polarizability is most effective at high frequencies, typically above 10 GHz. At these frequencies, the water molecules become polarized quickly, allowing the capacitor to store charge rapidly.
**Dipolar** polarizability in water occurs when the water molecules themselves have an inherent dipole moment, causing them to become polarized in response to the electric field of the capacitor. This type of polarizability is most effective at frequencies below 1 MHz, where the dielectric constant of water is low.
In conclusion, the dielectric constant of water is an important property that influences its behavior **as a resistor** in an electrical circuit. Understanding the resistance and capacitance of water in electrical circuits is important in designing and optimizing the performance of electrical systems that utilize water as a dielectric material. The relative permittivity of water in a capacitor depends on the frequency and temperature of the system. Different types of polarizability in water, such as **ionic**, **atomic**/**vibrational**, **electric**, and **dipolar**, are affected differently by different frequency ranges. Understanding these effects is important in designing and optimizing the performance of electrical systems that utilize water as a dielectric material.