Whenever one or more electrons are "**dislodged**" from the atom, the atom takes on a **net positive electrical charge** and is called a **positive ion**.
If an electron **combines** with a stable or normal atom, the atom has a **net negative charge** and is called a **negative ion**.
Voltage potential within an electrical circuit (see **Voltage Intensifier Circuit** as to Figure 1-1) can cause one or more electrons to be dislodged from the atom due to opposite polarity attraction between unlike charged entities, as shown in Figure (1-8).| **Voltage Intensifier Circuit** as to Figure 1-1 [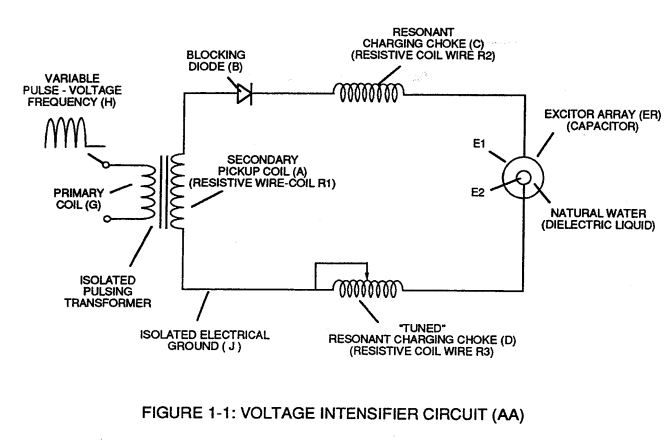](https://stanslegacy.com/uploads/images/gallery/2023-12/qhtqz9LzDjg4722f-image-1703011664617.png) | Figure (1-8) [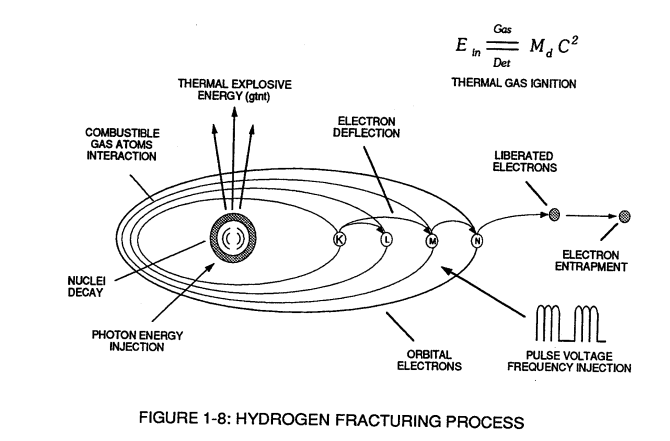](https://stanslegacy.com/uploads/images/gallery/2023-12/JwVGwSzunMi3kshP-image-1703014820006.png) |
The resultant electrical attraction force (qq') combines or joins unlike atoms together by way of **covalent bonding** to form molecules of gases, solids, or liquids.
[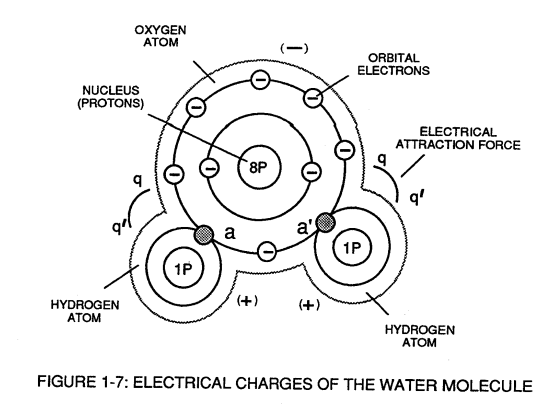](https://stanslegacy.com/uploads/images/gallery/2023-12/OPnNcyNwXCESXxxs-image-1703011752626.png)When the unlike oxygen atom combines with two hydrogen atoms to from the water molecule by accepting the hydrogen electrons (aa' of Figure 1-7), the oxygen atoms become "net" **negative electrically charged** (-) since the restructured oxygen atom now occupies 10 negative electrically charged electrons as to only 8 positive electrically charged protons. The hydrogen atom with only its **positive charged proton remaining** and **unused**, now, takes on a "net" **positive electrical charge** *equal to* the electrical intensity of the negative charges of the two **electrons** (aa') being shared by the oxygen atom. ... satisfying the law of physics that *for every action there is an equal and opposite reaction*. The sum total of the **two positive charged hydrogen atoms** (++) equaling the **negative charged oxygen atom** (--) forms a "no" net electrical charged molecule of water.Only the unlike atoms of the water molecule exhibits opposite electrical charges.