Whenever one or more electrons are "**dislodged**" from the atom, the atom takes-on a net positive electrical charge and is called a **positive ion**.
If a electron combines with a stable or normal atom, the atom has a **net negative charge** and is called a **negative ion.****Voltage potential** (65) within electrical **circuit** (60) can cause one or more **electrons** (79) to be dislodged from the **water molecule atom** (85) of Figure (3-26) due to **opposite electrical polarity attraction** (qq') of Figure (3-29) between unlike charged entities, as shown in (160) of Figure (326) as to **Newton's** and **Coulomb's laws** of **electrical-force**.
| Figure (3-26) [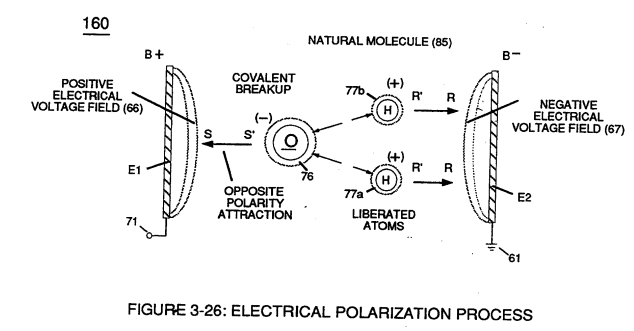](https://stanslegacy.com/uploads/images/gallery/2023-12/FI7iyHF5qFsbEw5X-image-1703376323691.png) | Figure (3-29) [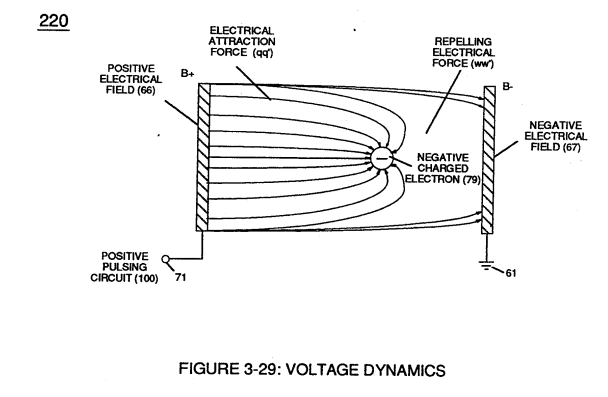](https://stanslegacy.com/uploads/images/gallery/2023-12/5AN8vz6fgBPasqR1-image-1703376333059.png) |
The resultant **electrical force** (qq') between the opposite electrical charged **hydrogen** (77) and **oxygen** (76) atoms keeps **water molecule** (210) intact when the **hydrogen atom** (77) shares its **electron** (84) with **oxygen atom** (76).
> The electrical strength of **attraction force** (qq') between the water molecule atoms is determined by the electrical size of the hydrogen atoms and the displacement of its **negative charged electrons** (84) during covalent sharing. [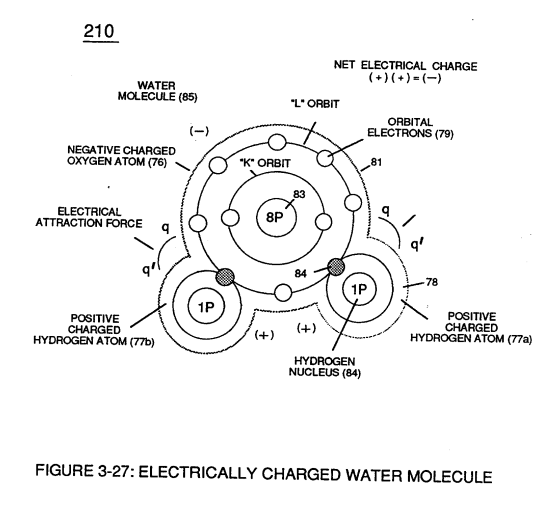](https://stanslegacy.com/uploads/images/gallery/2023-12/tgj1TAs4iQMFjqTd-image-1703376101819.png)Oxygen atom becomes **negative electrical charged** (81) since **oxygen atom** (76), now, has a total of ten negative charged **electrons** (79a xxx 79n) in its "K" plus "L" orbits while maintaining it's original **eight positive charged protons** which makes up **oxygen nucleus** (83).Since the **hydrogen proton** (84) (hydrogen nucleus) remains (after covalent link up), then the **hydrogen atom** takes-on a **positive charge** (78) co-equalling the **positive charge** of the **hydrogen nucleus proton** (84).
Together, the total net charge of **water molecule** (85) is zero despite the fact that each water molecule atom retains its electrical charge.
In other words, water molecule (85) is a electrically bipolar molecule having a stable configuration of charged atoms bound together by **electrostatic force** (qq').Electromagnetic bonding forces between unlike **atoms** (76n7) are negligible or non-existence, since **oxygen atom** (76) electrons are paired together, while rotating in opposite direction which, in turn, causes **oxygen atom** (76) to be electromagnetically neutral to **hydrogen atom** (77).
> Electron theory of magnetism requires orbital electrons to spin in the same direction before an atom can exhibit a electromagnetic field.Furthermore, **external electrical force** (66/67) can alter the electromagnetic properties of a atom since electromagnetic force is dependent on the movement of **charged particles** in a electrostatic field **voltage Intensifier circuit** (190) of figure (3-23), now, allows voltage to dissociates **water molecule** (85) by overcoming **electrostatic bonding force** (qq') between **unlike atoms** (76n7) while restricting amp flow, as illustrated in (160) of Figure (3-26).
| Figure (3-23) [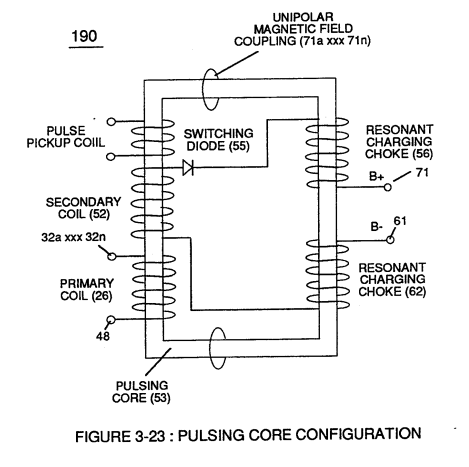](https://stanslegacy.com/uploads/images/gallery/2023-12/7ffVlnHJcIOSoYRS-image-1703232587555.png) | Figure (3-26) [](https://stanslegacy.com/uploads/images/gallery/2023-12/FI7iyHF5qFsbEw5X-image-1703376323691.png) |