Basics of Fuel Cells
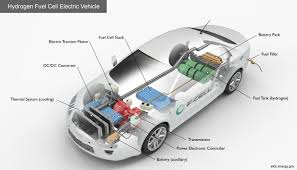
 Fuel cells are devices that convert chemical energy directly into electrical energy through an electrochemical reaction. Unlike conventional power generation methods, such as combustion engines that burn fuel to create heat and mechanical energy, fuel cells operate in a much cleaner and more efficient way. They rely on the reaction between hydrogen and oxygen, producing electricity, heat, and water as byproducts.
Fuel cells are devices that convert chemical energy directly into electrical energy through an electrochemical reaction. Unlike conventional power generation methods, such as combustion engines that burn fuel to create heat and mechanical energy, fuel cells operate in a much cleaner and more efficient way. They rely on the reaction between hydrogen and oxygen, producing electricity, heat, and water as byproducts.
The basic principle behind a fuel cell is similar to that of a battery, but with an important difference: while batteries store energy and need to be recharged or replaced once depleted, fuel cells continuously generate power as long as they have access to fuel and an oxidizing agent. This makes fuel cells appealing for a variety of applications, from powering vehicles to providing backup power for buildings and remote locations.
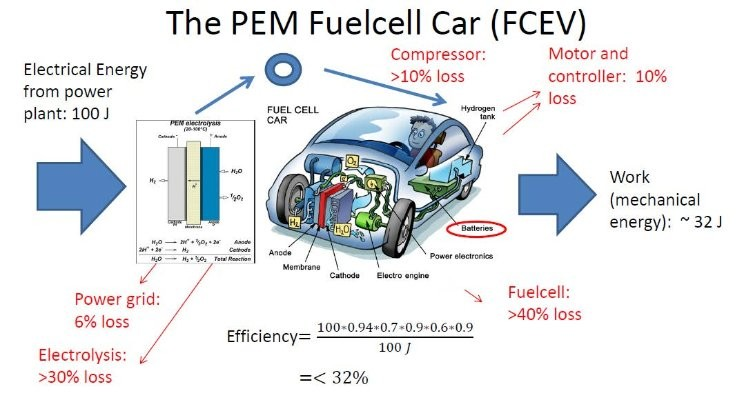
However, mainstream hydrogen fuel cells have significant limitations that are often overlooked. Traditional fuel cells require pure hydrogen gas, which is typically produced through energy-intensive processes such as steam methane reforming or water electrolysis. These processes not only consume large amounts of energy but also rely heavily on fossil fuels, thereby negating many of the supposed environmental benefits of fuel cells. Additionally, the need for specialized infrastructure to produce, store, and transport hydrogen adds complexity and cost, making mainstream fuel cells less practical as a widespread solution.
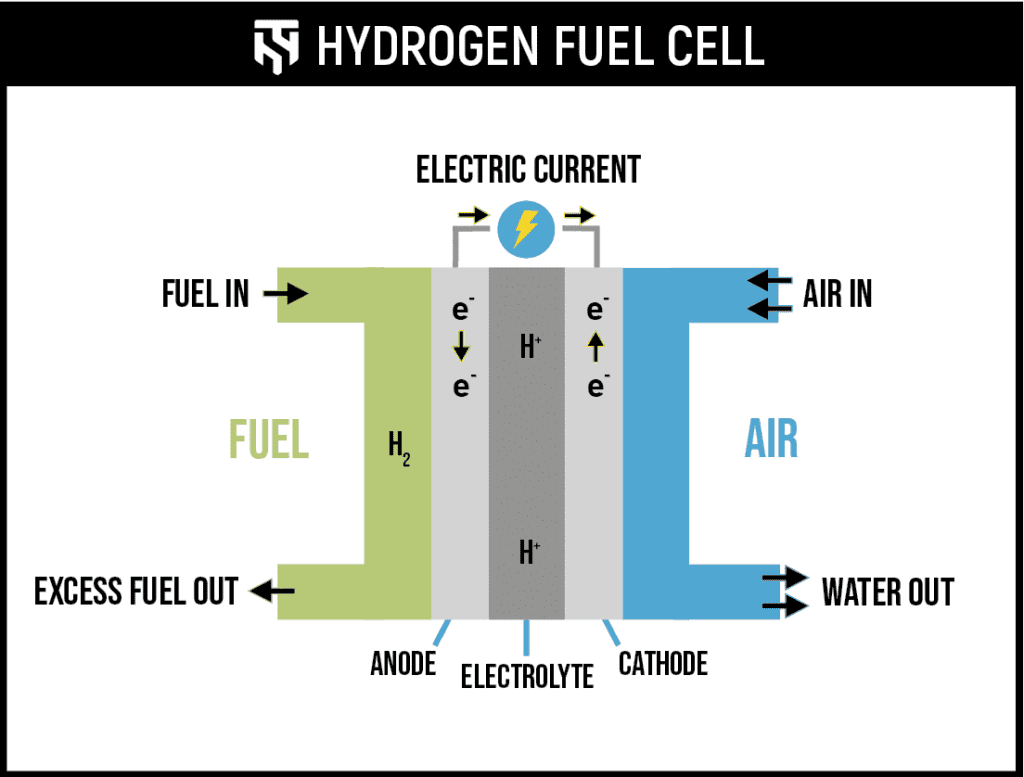
Fuel cells typically consist of three main components: the anode, the cathode, and an electrolyte that lies between them. Hydrogen gas is supplied to the anode, where it is split into protons and electrons. The electrons are forced through an external circuit, generating electricity, while the protons pass through the electrolyte to the cathode. At the cathode, oxygen from the air combines with the electrons and protons to produce water. This reaction is clean at the point of use, producing no harmful emissions. However, the overall lifecycle emissions and environmental impact depend heavily on how the hydrogen fuel is produced.
There are several types of fuel cells, each suited for different purposes. The most common types include Proton Exchange Membrane Fuel Cells (PEMFC), Solid Oxide Fuel Cells (SOFC), and Alkaline Fuel Cells (AFC). PEM fuel cells, for example, are popular in automotive applications because of their quick start-up time and compact size, whereas SOFCs are typically used for stationary power generation due to their higher efficiency and ability to use different types of fuels. Despite their varying applications, all of these fuel cells share the same fundamental drawbacks related to hydrogen production and infrastructure.
Stan's Process
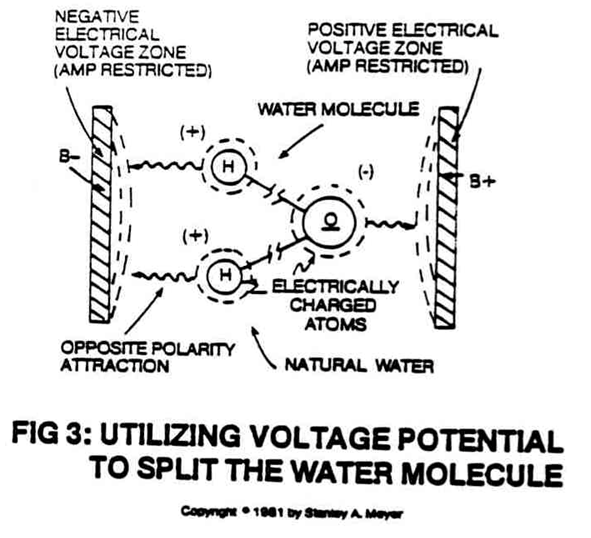
Stanley Meyer’s work focused on developing a radically different type of fuel cell—one that used water as the primary fuel source. Unlike conventional hydrogen fuel cells, which require hydrogen gas extracted through energy-intensive processes, Meyer aimed to split water molecules directly into hydrogen and oxygen using an innovative approach. His “water fuel cell” was designed to use high-voltage pulses to break the chemical bonds of water more efficiently, making it possible to generate hydrogen on-demand without the need for external hydrogen production infrastructure.
Meyer’s water fuel cell technology stood apart from traditional fuel cells in its attempt to produce hydrogen from water with minimal energy input. He envisioned a future where vehicles and other devices could run on water alone, making energy more accessible and reducing our dependence on fossil fuels. Mainstream fuel cells, by contrast, are hampered by their reliance on centralized hydrogen production and distribution, which limits their practical application as a truly decentralized energy solution.
While Meyer’s claims were met with skepticism, the idea of using water as a fuel continues to inspire researchers and alternative energy enthusiasts to explore new possibilities for clean and sustainable power generation. His approach promised to bypass many of the obstacles faced by mainstream fuel cells, offering a vision of energy independence and sustainability that goes far beyond the current limitations of hydrogen-based technologies.
Fuel cells, whether traditional or experimental like Meyer’s, hold great promise for the future of energy. However, it is essential to critically evaluate the challenges associated with conventional hydrogen fuel cells, including their reliance on fossil fuels and complex infrastructure. Understanding the basics of how fuel cells work, along with their limitations, provides an important foundation for grasping the significance of Meyer’s contributions to alternative energy technology.