EP0101761A2 Controlled Hydrogen Gas Flame
Europäisches Patentamt
European Patent Office
Office européen des brevetsPublication number: 0 101 761 A2
Source: SMeyer-EP0101761A2-Controlled_Hydrogen_Gas_Flame.pdf
EUROPEAN PATENT APPLICATION
- Application number: 82111597.9
- Date of filing: 14.12.82
- Priority: 25.08.82 US 411977
- Date of publication of application: 07.03.84 Bulletin 84/10
- Designated Contracting States: AT BE CH DE FR GB IT LU NL SE
Applicant: Meyer, Stanley A.
3792 Broadway
Grove City Ohio 43123 (US)Inventor: Meyer, Stanley A.
3792 Broadway
Grove City Ohio 43123 (US)Representative: Wassmiller, Alfons, Dipl.-Ing. et al.
Postfach 322 Gräfingerstrasse 7
D-8400 Regensburg (DE)
(54) Controlled hydrogen gas flame.
(A) A sustained controllable gas flame.
The hydrogen generator utilized is that for separating gasses from water having impurities and other gasses entrapped therein.
The gasses separated from the water comprises hydrogen, oxygen, and the non-combustible gasses, such as nitrogen.
The nitrogen, oxygen, and hydrogen are mixed as they are released in the process by the generator and collected as the mixture of gasses in the collection chamber of the generator.
The method and system comprises a nozzle of a given configuration connected through a line to the uppermost region of the gas collection chamber of the hydrogen generator.
The nitrogen reduces the velocity and temperature of the burning flame from that of the hydrogen/oxygen mixture.
To further control the temperature and velocity of the burning gas mixture there is added to the collection chamber other non-burnable gasses.
The configuration of the nozzle and its port opening is dependent on the mixture of gasses utilized and restricted thereby.
An increase in the size of the flame requires additional port openings to prevent blowout.
CROSS REFERENCE:
The hydrogen/oxygen generator utilized in the present invention is that disclosed and claimed in my co-pending U.S. patent application, Serial Number: 302,807, filed: September 16, 1981, for: HYDROGEN GENERATOR SYSTEM.
In that process for separating hydrogen and oxygen atoms from water having impurities, the water is passed between two plates of similar non-oxidizing metal.
No electrolyte is added to the water.
The one plate has placed thereon a positive potential and the other a negative potential from a very low amperage direct-current power source.
The sub-atomic action of the direct current voltage on the non-electrolytic water causes the hydrogen and oxygen atoms to be separated — and similarly other gasses entrapped in the water such as nitrogen.
The contaminants in the water that are not released are forced to disassociate themselves and may be collected or utilized and disposed of in a known manner.
The direct current acts as a static force on the water molecules; whereas the non-regulated rippling direct current acts as a dynamic force. Pulsating the direct current further enhances the release of the hydrogen and oxygen atoms from the water molecules.
 |
Controlled Hydrogen Gas Flame
PRIOR ART:
The electrolysis process for generating hydrogen and oxygen gas is well known in the art. It is, of course, further understood with a proper mixture of oxygen gas, the hydrogen gas is combustible and under ideal conditions a flame may be had. Reference is made to U.S. Patent Number: 4,184,921.
However, in that the burning velocity of hydrogen is 265-325 cm./sec. versus 37-45 cm./sec. of that of gasoline, the velocity of hydrogen is so great that the hydrogen ensuing from a nozzle will not under ordinary circumstances sustain a flame.
Therefore, to sustain a flame at a nozzle attached to a hydrogen generator the burning velocity of the hydrogen gas must be reduced.
It has been found that all water in its natural state, whether it be tap water, well water, sea water, or fresh water, is a saturation of ambient air.
Further, in that ambient air contains a substantial amount of nitrogen, all natural water will have entrapped therein nitrogen.
Again, the percentage of nitrogen entrapped in natural water has been determined to be a fixed percentage and very uniform at seventeen (17%) percent --- irrespective of the source of the water or its impurities.
Hence, a natural water gas analysis will show a seventeen percent of nitrogen relative to the hydrogen and oxygen.
The nozzle connected to the collection chamber via an appropriate line has a port opening of a controlled size and configuration, related to the size of the flame and the temperature and velocity of the burning gas mixture.
To maintain the flame, that is to prevent blowout, additional nozzles are included when the overall flame size is to be increased.
OBJECTS:
-
It is accordingly a principal object of the present invention to provide a new and improved hydrogen/oxygen generator that is operable from a water source that provides hydrogen/oxygen output that will have a sustained burn.
-
Another object of the present invention is to provide a hydrogen/oxygen generator that in addition to the hydrogen and oxygen gasses releases non-combustible nitrogen gas capable of reducing the burning velocity and temperature of a pure hydrogen/oxygen flame.
-
A further object of the present invention is to provide a hydrogen generator that includes the controlled addition of other non-combustible gasses to the gas chamber thereof to thereby further control the burning velocity and temperature of the hydrogen gas.
Other objects and features of the present invention will become apparent from a reading of the detailed description of the preferred embodiment taken in conjunction with the single figure drawings in which:
BRIEF DESCRIPTION OF THE DRAWINGS:
-
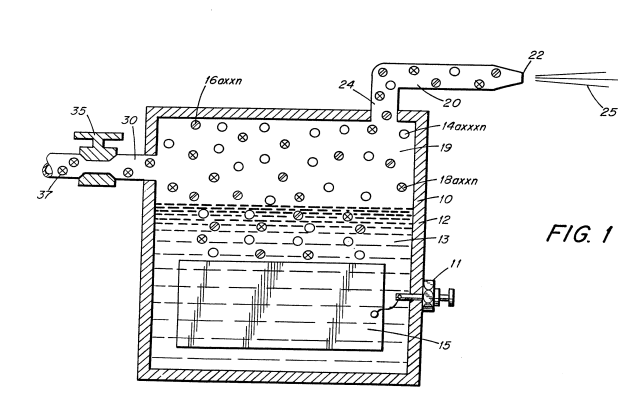
Figure 1 is a cross-section of a hydrogen generator illustrating the features of the present invention in its most preferred embodiment incorporated therein.
-
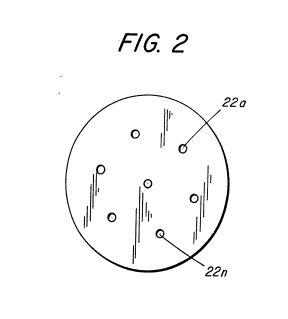
Figure 2 schematically shows the increased number of nozzle ports to increase the flame size.
 |
DETAILED DESCRIPTION OF DRAWINGS:
The hydrogen generator 10 is that of my co-pending patent application, supra. This generator comprises a closed watertight housing 12 having therein natural water 13.
Submerged in the water 13 is a pair of plates 15 (one not shown) having a direct current low amperage voltage, via connector 11, applied thereto.
As set forth in my co-pending patent application, supra, the electrical potential applied to the similar non-oxidizing metal plates is a sub-atomic action.
In this way, the hydrogen atoms 14 and the oxygen atoms are released from the water molecule.
Unlike the electrolysis process for generating hydrogen from distilled water, the hydrogen generator of my aforementioned patent application utilizes water 13 that need not be pure --- simply any water irrespective of contaminants and source.
Natural water such as tap, well, sea, or fresh water is an absorber of ambient air.
Ambient air in turn contains a substantial amount of nitrogen gas.
Water as an absorber of ambient air will entrap seventeen percent (17%) of nitrogen gas; that is, natural water absorbs seventeen percent (17%) of nitrogen gas in comparison to its hydrogen and oxygen gas content.
In operation of the hydrogen generator, in that it is a subatomic or force-type of generator, the gasses in the water will be released.
Therefore, when natural water is used the nitrogen gas will be released together with the hydrogen and oxygen gasses.
In the preferred embodiment utilizing tap water, the nitrogen gasses 16 are intermixed with the hydrogen gasses 14 and the oxygen gasses 18 in the chamber 19 of the hydrogen generator 10.
Upon release of the gasses via line 24 and nozzle 20 and then port 22 the gas mixture is ignited to provide flame 25.
The flame 25 is sustained in that the nitrogen gasses 16 reduce the burning velocity and temperature of the hydrogen gas 14.
A realistic and practical manner of further controlling the burning velocity and temperature of the hydrogen gasses 14 is by adding non-combustible gasses directly to the hydrogen and oxygen gasses generated. This is accomplished
by inlet 30 to the upper gas chamber 19 of the hydrogen generator. Valve means 35 is adjustable to control the amount of non-combustible gasses added to the gas chamber.
The nozzle 20 connected to the chamber 19 of the generator 10 via line 17, is of a given configuration to permit a predetermined quantity of gasses to be expelled from the port 22. The port size is dependent on the gasses generated, and collected in the chamber 19, the pressure of the chamber 19 of the generator 10, and the size of the flame desired.
To increase the size of the flame 25 would appear to be a simple matter of increasing the rate of gasses generated. However, an increase of gasses merely causes a blowout at the port 22 opening of the nozzle 20. This flame blowout will occur since an increase in hydrogen gas generation disrupts the ratio of the initial mixture, even though the percentages remain constant. Typically, tap water will contain 62% hydrogen, 31% oxygen, and 17% nitrogen. In actuality, the percentages may be somewhat less dependent on other gasses that may be trapped in the tap water. The increase in production will not affect the percentages, but it must be appreciated that the volume of the gasses will be proportionately increased. In turn, the volume being directly related to pressure, the pressure will be similarly increased.
To effectively reduce or counter the velocity due to the increased pressure of the hydrogen gas mixture in the chamber 19, a larger port 22 would appear to be capable of handling the increased pressure. But, as aforesaid, a larger port end and the concentration of the high velocity hydrogen gas mixture will cause a flame blowout. To sustain a larger flame with increased pressure, additional nozzles having ports or a nozzle 20 with multiple ports as shown in Figure 2, of a port size predetermined as aforesaid, will be added to the line 17. Accordingly, the larger the desired flame, the greater the number of ports.
It can be understood that a port that will not sustain a flame does present a safety factor relative to hydrogen spark back to the chamber 19. Hence, controlling the size of the port 22 in effect acts as a quencher of hydrogen spark back.
Although certain and specific embodiments are shown and described it is within the scope and spirit of the present invention to include alternatives and modifications thereto.
CLAIMS:
-
A hydrogen/oxygen generator capable of sustaining the controlled burning of the gasses generated thereby comprising:
- a housing having natural water therein including entrapped non-combustible gasses,
- a pair of similar non-oxidizing plates having direct current low amperage voltage applied thereto to provide a sub-atomic, force-type action on said water,
- said action liberating the hydrogen atoms and oxygen atoms from said water molecule, and further liberating said non-combustible gasses from said water,
- a gas collection chamber in said generator for collecting and intermixing said released gasses,
- a nozzle attached to the gas collection chamber of said housing including an inlet for receiving the mixture of hydrogen, oxygen and non-combustible gas,
- said nozzle of a predetermined size and configuration on a port for expelling said mixed gasses, and means for igniting said mixed gasses.
-
The hydrogen/oxygen generator of Claim 1 wherein said non-combustible gas is nitrogen.
-
The hydrogen/oxygen generator of Claim 2 wherein said gasses intermixed in said collection chamber of the hydrogen generator comprise 62% hydrogen, 31% oxygen, and 17% nitrogen.
-
The hydrogen/oxygen generator of Claim 3 wherein said means comprises an inlet connected to said gas collection chamber of said housing, and means for introducing non-combustible gasses to said chamber.
-
The hydrogen/oxygen generator of Claim 4 wherein said inlet further comprises valve means for controlling the amount of non-combustible gasses introduced to said chamber.
-
The hydrogen/oxygen generator of Claim 5 wherein said port on said nozzle of a predetermined size and configuration is related to the ratio of gasses in said collection chamber to provide a flame of a predetermined velocity and size at said port.
-
The hydrogen/oxygen generator of Claim 6 wherein said port size and configuration is maintained with a plurality of ports to thereby permit a proportional increase in flame size.