CA1235669A1 Controlled Hydrogen Gas Flame
PDF Download: SMeyer-CA1235669A1-Controlled_Hydrogen_Gas_Flame.pdf
Consumer and Consornmation aS Corporate Affairs Canada el Corporations Canada
(11) (A) No. 1 235 669
(45) ISSUED 880426
(52) CLASS 204-78 C.R. CL. 204-1664
(51) INT. CL. c25B 1/04us ca GANADIAN PATENT «2
(54) Controlled Hydrogen Gas Flame
(72) Meyer, Stanley A.,U.S.A.
(21) APPLICATION No. 420,890
(22) FILED 830204
(30) PRIORITY DATE U.S.A. (411,977) 820825 No. OF CLAIMS 9Canada
DISTRIBUTED BY THE PATENT OFFICE, OTTAWA, CCA-274 (11-82) VX
Illustrations
 |
ABSTRACT
A sustained controllable gas flame. The hydrogen generator utilized provides gasses from water having impurities and other gasses entrapped therein. The hydrogen generator has a chamber for holding a quantity of water and a pair of non-oxidizing electrodes are located in the chamber. A rippling wave form electric potential is applied across the electrodes without any change of polarity and the current flow is limited. The gasses separated from the water comprise combustible gasses such as hydro- gen and oxygen and non-combustible gasses, such as nitrogen. The nitrogen, oxygen and hydrogen are mixed as they are released in the process and collected as a mixture of gasses in a collection chamber of the generator. A nozzle having one or more ports of a given configuration is connected through a line to the uppermost region of the gas collection chamber of the hydrogen generator. The nitrogen reduces the velocity and temperature of the burning flame from that of the hydrogen/oxygen mixture. To further control the temperature and velocity of the burning gas mixture there ‘6 added to the collection chamber other non-burnable gasses. The configuration of the nozzle and its port opening for sustaining a flame is dependent on the mixture of gasses utilized and restricted thereby. An increase in the size of the flame requires additional port openings to prevent blowout.
This invention relates to apparatus for generating gases from water and for the controlled burning of such generated gases.
The present invention utilizes the hydrogen/oxygen generator disclosed and claimed in my co-pending Canadian Patent Application Serial No. 420,908 filed 4 February, 1983 and entitled "HYDROGEN GENERATOR SYSTEM".
In the process disclosed in said application for separating hydrogen and oxygen atoms from water having impurities, water is passed between two plates of similar non-oxidizing metal. No electrolyte is added to the water. The one plate has placed thereon a positive potential and the other a negative potential from a very low amperage direct— current power source. The action of the direct current voltage on the non-electrolytic water causes the hydrogen and oxygen atoms to be separated and also released are other gases entrapped in the water such as nitrogen. Contaminants in the water that are not released as gases are forced to separate from the water and may be collected or utilized and disposed of in a Known manner.
Direct current acts as a static force on the water molecules and a non-regulated rippling direct current acts as a dynamic force. Pulsating the direct current further enhances the release of the hydrogen and oxygen atoms from the water molecules,
The electrolysis process for generating hydrogen and oxygen gas from water is well known in the art. It is, of course, further understood with a proper mixture of oxygen gas, the hydrogen gas is combustible and under ideal conditions a flame, may be had.
Reference is made to U.S. Patent 4,184,921. However, in that the burning velocity of hydrogen is so great (265-325 cm./sec. versus 37-45 cm./sec. for gasoline) the hydrogen ensuing from a nozzle will not under ordinary circumstances sustain a flame.
Therefore, to sustain a flame at a nozzle attached to a hydrogen generator the burning velocity of the hydrogen gas must be reduced.
It has been found that all water in its natural state whether it be tap water, well water, sea water, or fresh water is a saturate of ambient air. Further, in that ambient air contains a substantial amount of nitrogen, all natural water will have entrapped therein nitrogen. Again, the percentage of nitrogen entrapped in natural water has been determined to be a fixed percentage and very uniform irrespective of the source of the water or its impurities.
A principal object of the present invention is to provide a new and improved hydrogen/oxygen generator that is operable from a water source that provides hydrogen/oxygen output that will have a sustained burn.
Another object of the present invention is to provide a hydrogen/oxygen generator that in addition to the hydrogen and oxygen gases releases non-combustible nitrogen gas capable of reducing the burning velocity and temperature of a pure hydrogen/oxygen flame.
A further object of the present invention is to provide a hydrogen generator that includes the controlled addition of other non-combustible gases to the gas chamber thereof to thereby further control the burning velocity and temperature of the hydrogen gas.
In accordance with the present invention a nozzle is connected to a gas collection chamber via an appropriate line and has a port opening of a controlled size and configuration, related to the size of the flame and the temperature and velocity of the burning gas mixture. To maintain the flame, i.e. prevent blowout, additional nozzles are included when the overall flame size is to be increased.
More particularly there is provided in accordance with the present invention an apparatus for generating gases from water and for the controlled burning of the gases generated thereby comprising:
a housing having a chamber for containing natural water therein including entrapped non-combustible gases;
a pair of similar non-oxidizing electrodes in said chamber and adapted to be connected to a source of rippling wave form direct current voltage in which the current flow is restricted to provide a force action on water in said chamber, which liberates the hydrogen atoms and oxygen atoms from said water as well as non-combustible gases dissolved in said water and thereby producing a mixture of hydrogen, oxygen and non— combustible gases;
a gas collection chamber in said housing for collecting and intermixing said released gases; and
a nozzle attached to the gas collection chamber of said housing including an inlet for receiving said mixture of hydrogen, oxygen and non-combustible gases;
said nozzle having a port of predetermined size and configuration for expelling said mixed gases at a rate so as to sustain a flame from the gases that are liberated from the water.
Description Of Drawings:
The invention is illustrated by way of example in the accompanying drawings wherein:
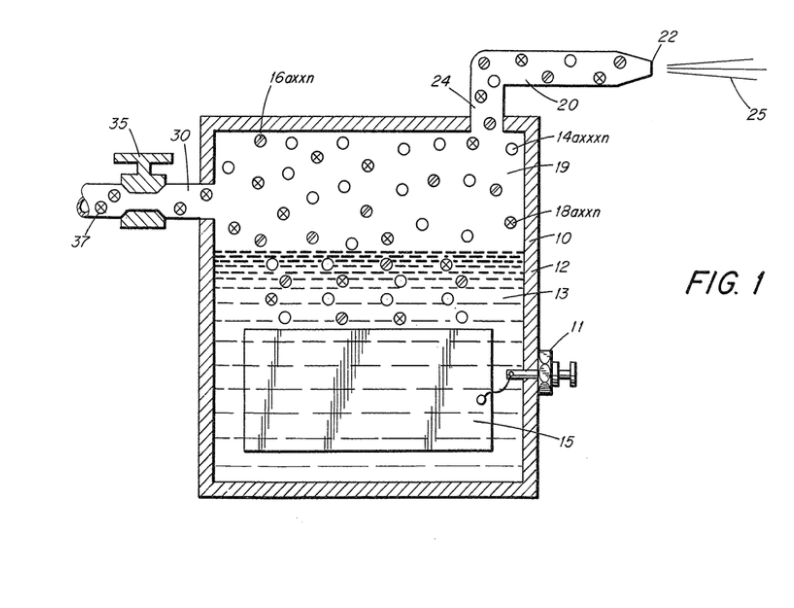
Figure 1 is a cross-sectional view of a hydrogen generator illustrating the features of the present invention in. its most preferred embodiment incorporated therein; and
Figure 2 is a front face view of a nozzle having increased number of nozzle ports to increase the flame size.
Referring to the drawings the hydrogen generator 10 is the subject of my aforementioned co-pending application Serial No. 420,908. The generator 10 comprises a closed watertight housing 12 having a quantity of natural water 13 in the bottom thereof. Submerged in the water 13 is a pair of plates 15 (one not shown) which have a direct current low amperage voltage, applied thereto via connector 11 during operation. As get forth in my co-pending patent application supra, the electrical potential applied to the similar non-oxidizing metal plates is a sub-atomic action. In this way the hydrogen atoms 14 a xxx n and the oxygen atoms 18 a xxx n are released from the water molecule.
Unlike the electrolysis process for generating hydrogen from distilled water, the hydrogen generator of my aforementioned patent application, utilizes water 13 that need not be pure --~ simply any water irrespective of contaminants and source.
Natural water such as tap, well, sea, or fresh water is an absorber of ambient air and since ambient air contains a substantial amount of nitrogen gas so does the natural water with the ambient air absorbed therein. In operation of the hydrogen generator, the gases released from natural water are thus nitrogen, hydrogen and oxygen.
In the preferred embodiment utilizing tap water, the nitrogen gasses 16 a xxx n are intermixed with the hydrogen gases 14 a xxx n and the oxygen gases 18 a xxx n in the chamber 19 of the hydrogen generator 10. Upon release of the gases via line 24 and nozzle 20 and then port 22 the gas mixture is ignited to provide flame 25.
The flame 25 is sustained in that the nitrogen gases 16 a xxx n reduces the burning velocity and temperature of the hydrogen gas 14 a Xxx Nn.
A realistic and practical manner of further controlling the burning velocity and temperature of the hydrogen gases 14 a xxx n, is by adding non-combustible gases directly to the hydrogen and oxygen gases generated. This is accomplished by inlet 30 to the upper gas chamber 19 of the hydrogen generator. Valve means 35 is adjustable to control the amount of non- combustible gases 37, added to the gas chamber 19.
The nozzle 20 connected to the chamber 19 of the generator 10 via line 17, is of a given configuration to permit a predetermined quantity of gases to be expelled from the port 22. The port size is dependent on the gases generated, and collected in the chamber 19, the pressure of the chamber 19 of the generator 10, and the size of the flame desired.
To increase the size of the flame 25 would appear to be a simple matter of increasing the rate of gases generated. However, an increase of gases merely causes a blowout at the port or opening 22 of the nozzle 20. This flame blowout will occur since an increase in hydrogen gas generation disrupts the ratio of the initial mixture even though the percentages remain constant. Typically, tap water contains known percentages of hydrogen, oxygen, and nitrogen. The percentages vary somewhat dependent on the other gases that may be trapped in the tap water. The increase in production will not affect the percentages but it must be appreciated that the volume of the gases will be proportionately increased. In turn, the volume being directly related to pressure, the pressure will be similarly increased.
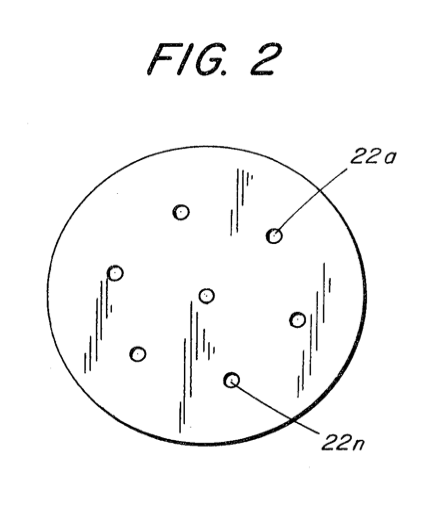 To effectively reduce or counteract the velocity due to the increased pressure of the hydrogen gas mixture in the chamber 19 a large port 22 would appear to be capable of handling the increased pressure, but as aforesaid, a larger port with a high velocity hydrogen gas mixture will cause a flame blowout. To sustain a larger flame with increased pressure, additional nozzles having ports 22 a xxx n or, a nozzle 20 with multiple ports 22 a xxx n as shown in Figure 2, can be used. The larger the desired flame the greater will be the number of ports required.
To effectively reduce or counteract the velocity due to the increased pressure of the hydrogen gas mixture in the chamber 19 a large port 22 would appear to be capable of handling the increased pressure, but as aforesaid, a larger port with a high velocity hydrogen gas mixture will cause a flame blowout. To sustain a larger flame with increased pressure, additional nozzles having ports 22 a xxx n or, a nozzle 20 with multiple ports 22 a xxx n as shown in Figure 2, can be used. The larger the desired flame the greater will be the number of ports required.
It can be understood that a port that will not 5 sustain a flame does present a safety factor relative to hydrogen spark back to the chamber 19. Hence, controlling the size of the port 22 in effect provides a quencher of hydrogen spark back.
Although certain and specific embodiments are shown and described it is within the scope and spirit of the present invention to include alternatives and modifications thereto.
THE EMBODIMENTS OF THE INVENTION IN WHICH AN EXCLUSIVE PROPERTY OR PRIVILEGE IS CLAIMED ARE DEFINED AS FOLLOWS:
1. An apparatus for generating gases from water and for the controlled burning of the gases generated thereby comprising:
a housing having a chamber for containing natural water therein including entrapped non-combustible gasses;
a pair of similar non-oxidizing electrodes in said chamber and adapted to be connected to a source of rippling wave form direct current voltage in which the current Flow is restricted to provide a force action on water in said chamber, which liberates the hydrogen atoms and oxygen atoms from said water as well as non-combustible gases dissolved in said water and thereby producing a mixture of hydrogen, oxygen and non-combustible gases;
a gas collection chamber in said housing for collecting and intermixing said released gases; and
a nozzle attached to the gas collection chamber of said housing including an inlet for receiving said mixture of hydrogen, oxygen and non-combustible gases;
said nozzle having a port of predetermined size and configuration for expelling said mixed gases at a rate so as to sustain a flame from the gases that are liberated from the water.
2. The apparatus of Claim 1 including means for separately introducing non-combustible gases to said gas collection chamber.
3. The apparatus of Claim 2 including valve means for controlling the amount of non-combustible gases introduced into said chamber.
4. The apparatus of Claim 1 wherein said nozzle has a plurality of separate ports to thereby control the flame.
5. A method of producing a combustible gaseous mixture and sustaining controlled burning thereof comprising: (a) providing a quantity of water in a water holding chamber having a pair of similar non-oxidizing electrodes therein; (b) applying a rippling wave form electric potential across said electrodes without any change of polarity and limiting the current flow so as to liberate hydrogen atoms and oxygen atoms from the water molecules as well as non-combustible gases dissolved in the water and thereby provide combustible and non-combustible gases; (c) collecting and intermixing said gases; and (ad) providing a nozzle having at least one port of predetermined size and configuration such that the gaseous mixture flows therethrough at a rate so as to sustain a flame.
6. The method of Claim 5 including the step of burning said gaseous mixture at said nozzle port.
7. The method of Claims 5 or 6 including the step of adding further non-combustible gases to said intermixed gases to provide a combustible gas mixture with a selected burn rate.
8. The method of Claim 7 including the step of burning said gaseous mixture at said nozzle port.
9. The method of Claims 7 or 8 including the step of adding further non-combustible gases to said intermixed gases to provide a combustible gas mixture with a selected burn rate.
