Hydrogen Gas Utilization
THE NEED TO RENDER HYDROGEN SAFE:
Not only can the Fuel-Cell economically produce. a huge amount of hydrogen gas on demand, it can also adjust the burn rate of the hydrogen gas to co-equal the burn rate of fossil fuels without additive chemicals.
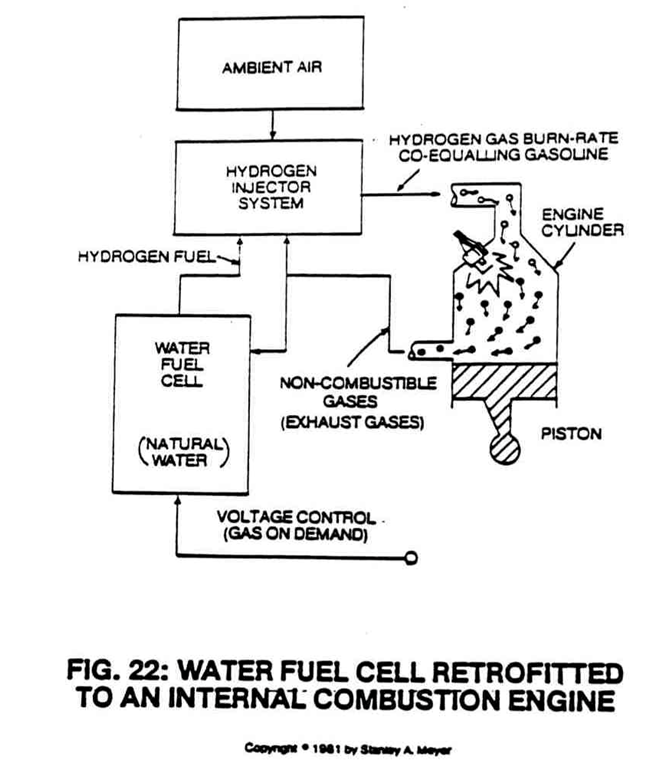
For example, to run a typical automobile on hydrogen gas without altering the engine or performance, the hydrogen gas is automatically adjusted to co-equal the burn rate of gasoline or diesel fuel, as shown in Figures 22 and 23.
 |
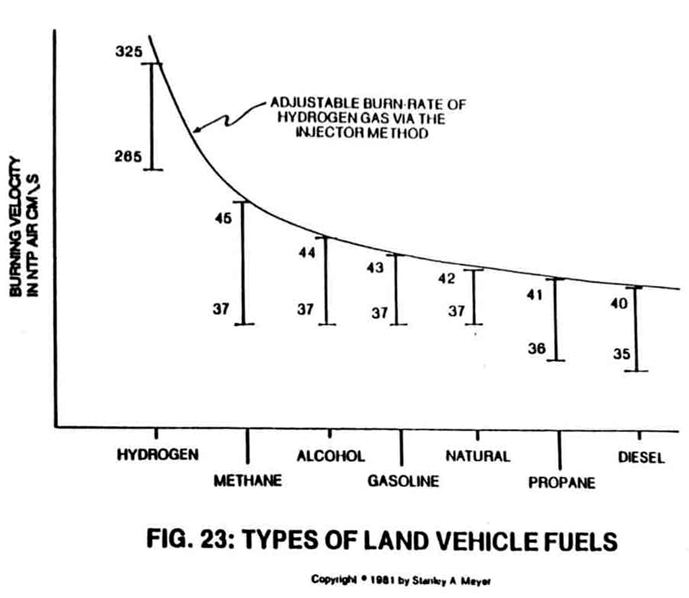 |
The Fuel Cell can also duplicate the burn rate of natural gas or other burnable fuels now being used in the marketplace.
The Fuel Cell simply has the ability to safely and effectively render hydrogen as a useful fuel and does it economically. It also means that the Fuel Cell can be retrofitted to any type of existing power system using gas fuel.
QUENCHING CIRCUIT: ANTI SPARK-BACK:
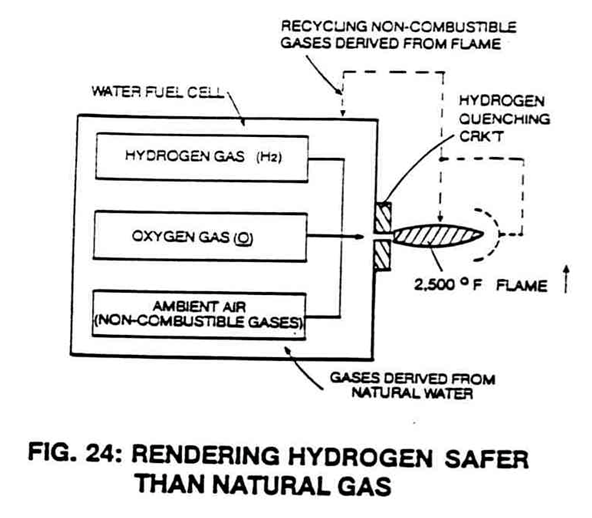
The Fuel Cell accomplishes this by using a hydrogen quenching circuit (see Figure 24) with the use of natural water.
This small, button-sized circuit allows for the adjusting of the burn rate to support a sustained hydrogen-oxygen flame well over 2,500 degrees Fahrenheit without any spark-back into the Fuel Cell.
It also helps keep the gases uniformly mixed inside the Fuel Cell during gas production.
SUSTAINED HYDROGEN FLAME ON DEMAND:
During the splitting of the water molecule, ambient air supplied by the water and having gases that do not support combustion passes through the very small quenching circuit with the hydrogen and oxygen gases prior to flame combustion.
The non-combustible gases act as a modulator that reduces the speed at which the oxygen atom unites with the hydrogen atom to cause flame combustion.
More specifically, the non-combustible gases associated with ambient air entrapped in natural water act as a dilutant that allows the hydrogen to burn at a rate equal to fossil fuels.
The water itself acts as a gas-mixing regulator where the gas-mixing ratio remains constant regardless of the rate of gas production.
Again, no costly manufacturing process is needed to convert hydrogen to fossil fuel burning levels.
If for some reason you need a flame temperature greater than 2,500 degrees F., simply reduce the amount of non-combustible gases being mixed with the hydrogen.
If you need the flame temperature to be lowered, simply recycle the expelled flame gases (ambient air gases exposed to the sustained hydrogen flame) back into the burning process.
These gases further dilute the hydrogen burn rate.
However, gas production is still dictated by demand, and mixing rate maintained regardless-of the rate of gas generation.
The hydrogen flame is sustained and maintained at all times during Fuel Cell operations.
HYDROGEN AS A CLEAR BURNING FUEL:
 The by-product of burning hydrogen and oxygen is simply water mist when the Fuel Cell utilizes a specially designed catalytic block that ensures almost one hundred percent conversion of the Fuel Cell burnable gases.
The by-product of burning hydrogen and oxygen is simply water mist when the Fuel Cell utilizes a specially designed catalytic block that ensures almost one hundred percent conversion of the Fuel Cell burnable gases.
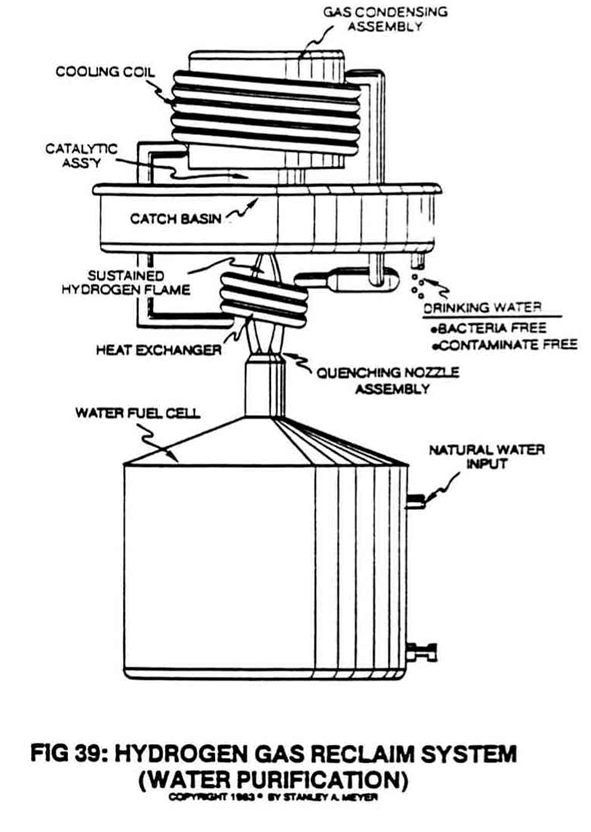
The catalytic block, as used in the hydrogen reclaim system of Figure 39, accomplishes this gas conversion process in a systematic way.
First, the catalytic block entraps all combustible gases (gases expelled from the flame) that have not undergone the flame burning process of combustion.
The catalytic block re-exposes the escaped an entrapped burnable oases to the sustained hydrogen flame for gas combustion...eliminating any and all unused hydrogen and oxygen atoms...by combining these free-floating atoms to form water vapor when ignited.
This second stage burning process superheats the catalytic block to further aid the combustion process.
Once the oxygen and hydrogen atoms are completely locked into the water molecule formation, the atoms are consumed and cannot unite with other ambient air gases.
For example, Nitrous Oxide (N20) cannot form since there are no oxygen atoms available to unite with the non-combustible nitrogen gas to form an oxide gas.
Likewise, Nitrous Acid (HNO2) in solution cannot form since there are no hydrogen atoms available to unite with nitrogen and oxygen atoms to form an acid in solution.
Remember, there are no oxygen atoms present to help form the acid base.
The catalytic block simply renders the Fuel Cell environmentally safe.
This simply means that the water vapor produce from the Fuel Cell can be recycled back into the atmosphere to form rain which can be collected again for hydrogen gas reuse.
Of course, this recycling method is not as efficient as the hydrogen gas reclaim system of Figure 39, and so, illustrated in Figure A.
QUENCHING TUBE: DISTRIBUTING HYDROGEN SAFELY:
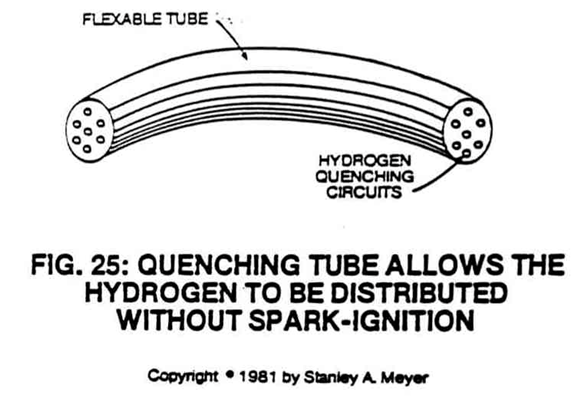 To transport hydrogen gas from the Water Fuel Cell without spark ignition, the quencher circuit is carried one step further. Transportation of hydrogen--- is done without regard for distance, through the "quenching tube" see Figure 25.
To transport hydrogen gas from the Water Fuel Cell without spark ignition, the quencher circuit is carried one step further. Transportation of hydrogen--- is done without regard for distance, through the "quenching tube" see Figure 25.
As the hydrogen passes through the tube, ignition cannot occur because it is impossible for the hydrogen, oxygen, and non-combustible gases to unite within the small confines of what is essentially an elongated quenching circuit.
Gas pressure is not a factor either.
If the gas pressure from the Fuel Cell decreases or is somehow shut off, the hydrogen still cannot retrace its path with the oxygen to unite for combustion.
FUEL CELL MEETS SAFETY REQUIREMENTS:
The Fuel Cell is basically a composite of many systems integrated into a functional system.
The system is one of constant demand that stores hydrogen in water until used.
Water is the safest storage medium known to man.
The hydrogen burn rate is adjusted to co-equal that of fossil fuels by using the quenching circuit and recycling of non-combustible gases back into the system.
This allows hydrogen gas to be used in place of natural gas.
The hydrogen gas can be transported without spark ignition, thanks to the quenching tube.
The burning of the hydrogen gas is rendered clean by the catalytic process.
And finally, hydrogen recycling is performed for energy reuse.
These integrated features give the Fuel Cell the ability to comply with and supersede any and all federal, state, and local housing and/or highway safety regulations.