The Principles of Discovery
In order to conform with the development criteria, it is clear that hydrogen gas must be produced economically—without the use of exotic materials or complicated processes. The question loomed before Stan Meyer of how to easily separate the hydrogen from the oxygen in the water molecule. If the atoms of the water molecule could easily be disassociated, hydrogen would be a cheap and abundant fuel source.
THE PURSUIT OF KNOWLEDGE:
Meyer dealt with the prospect of how to capitalize upon the natural potential held by the hydrogen and oxygen atoms which keep the water molecule bound together. He discovered the simple yet profound principle, ELECTRICAL POLARIZATION OF THE WATER MOLECULE.
ELECTRICALLY CHARGING THE WATER MOLECULE:
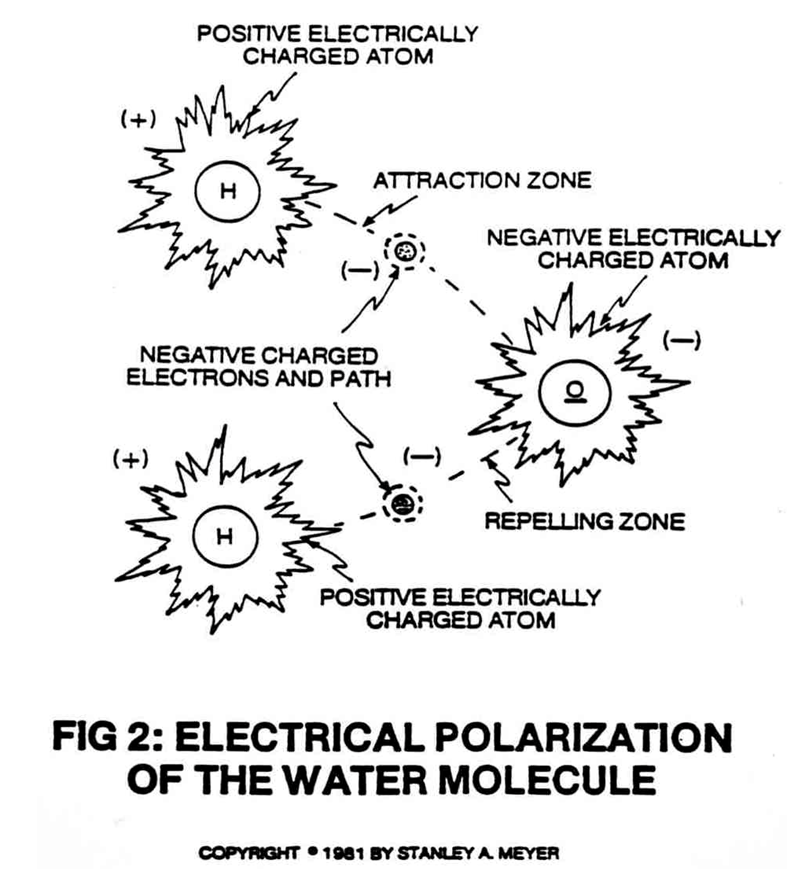 It is already known that the hydrogen and oxygen atoms by themselves may take on electrical charges.
It is already known that the hydrogen and oxygen atoms by themselves may take on electrical charges.
But until now, no one discovered that by simultaneously exposing the water molecule to one positively charged and one negatively charged electrical voltage zone, the unlike hydrogen and oxygen atoms assume opposing electrical characteristics, equal in magnitude and potential, thereby stabilizing the electrical polarity of the water molecule into existence. In the water molecule, the two hydrogen atoms take on a positive (+) electrical care and the one oxygen atom takes on a negative (-) electrical charge, thereby satisfying the two basic laws of physics...for every action there is an equal and opposite reaction, and that all things must reach a stable state, as so illustrated in Figure 2.
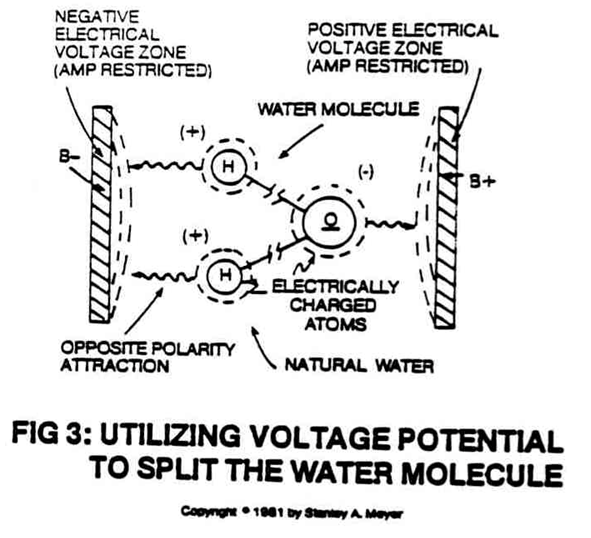 Although the molecule, is stabilized, in electrical polarity by the simultaneous application of the two oppositely charged voltage zones, the bond between its atoms is greatly weakened in this process.
Although the molecule, is stabilized, in electrical polarity by the simultaneous application of the two oppositely charged voltage zones, the bond between its atoms is greatly weakened in this process.
The positively charged hydrogen atoms are attracted to the negatively charged voltage zone, and the negatively charged oxygen atoms are attracted to the positively charged voltage zone...satisfying the opposite polarity attraction law of physics as shown in Figure 3.
ELECTRICAL POLARIZATION PROCESS:
Simply, the electrical polarization of the water molecule is basically a four-step process.
- First, oppositely charged electrical voltage zones are simultaneously introduced to either side of the water molecule.
- Secondly, the water molecule becomes electrically polarized (electrically charged).
- Thirdly, this electrical polarization of the hydrogen and oxygen atoms greatly weakens the stability of the water molecule.
- And, finally, because the-voltage zones are still present with their opposite electrical attractions, the water molecule is split into it component parts.
The hydrogen and oxygen atoms separate, with the hydrogen being attracted to the negative electrical voltage zone, while the oxygen is attracted to the positive electrical voltage zone…all simultaneously.
COVALENT BREAK-UP OF THE WATER MOLECULE:
In scientific terms, once the electrical polarization process occurs, the covalent bonding or sharing of electrons between atoms of the water molecule ceases to exist since the positive electrically charged hydrogen atoms attract the free floating negative charged electrons; while, at the same time, the negative electrically charged oxygen atom repels the moving electron...
- thereby stabilizing the energy level of the atoms...
- separating the water molecule into its component parts...
- releasing energy in the form of hydrogen gas and oxygen gas, as illustrated in Figure 3.
The opposite polarity attraction that now exists between the liberated electrically charged atoms and the stationary electrical voltage zones further aids the splitting process. The repetitive pulse voltage frequency potential, or reforming voltage zones, continue the electrical polarization process.
What is so dramatic about disassociation of the atoms in the water molecule is the ease at which the task is done. Because the bond between the hydrogen and oxygen is already greatly weakened by the electrical polarization of the water molecule, very little energy in the form of applied voltage is needed to actually separate the hydrogen and oxygen atoms. The hydrogen and oxygen atoms that make up the water molecule are already seeking to move in opposite directions because of their respective attractions to the opposite electrical voltage zones. A minute amount of voltage, a potential energy without amp consumption, gives the atoms the impetus to break away from one another in a strictly physical process. Hydrogen and oxygen gases are released in great amounts with little energy being consumed and without chemical interaction. Once the splitting of the molecule occurs, the liberated hydrogen and oxygen atoms will not recombine in the polarization process. Therefore, the gas can be utilized for energy consumption.
NATURAL WATER, THE SOURCE FOR HYDROGEN:
Under actual lab certification testing, it is shown that by utilizing pulse voltage frequency potential, splitting of the water molecule occurs in all natural water, even distilled water, as shown in Figure 4.