The Silver Coulometer
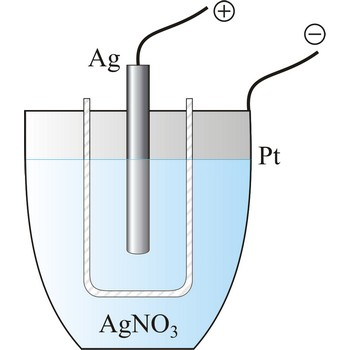
A silver coulometer is a device used to measure the quantity of electricity (in coulombs) by observing the amount of silver deposited or dissolved in a solution. Here's how voltage influences ions in the operation of a silver coulometer:
-
Basic Principle: The silver coulometer is based on Faraday's laws of electrolysis. When an electric current passes through a solution of silver nitrate (AgNO₃), silver ions (Ag⁺) are reduced to silver metal (Ag) at the cathode, and the corresponding amount of silver is deposited.
-
Voltage Influence:
- Initiation: For electrolysis to start, the applied voltage must overcome the combined potential of the silver/silver ion electrode and any resistance in the solution. This is called the decomposition potential.
- Controlled Rate: The voltage applied across the electrodes influences the rate at which silver ions are reduced and deposited. A higher voltage increases the current, which, according to Faraday's laws, increases the rate of deposition of silver.
-
Electrode Reactions: At the cathode, where reduction occurs, silver ions (Ag⁺) gain an electron to become silver atoms (Ag). At the anode, silver atoms lose an electron to become silver ions. The voltage ensures these reactions occur continuously and at a steady rate.
-
Faraday’s Laws:
- First Law: The mass of a substance altered at an electrode during electrolysis is proportional to the quantity of electricity that passes through the electrolyte. This means more voltage (and thus more current for a given resistance) will deposit more silver.
- Second Law: The masses of different substances liberated by the same quantity of electricity passing through the electrolytic solution are proportional to their chemical equivalent weights.
-
Measurement: By measuring the mass of silver deposited at the cathode, and knowing the constant that relates charge to the mass of silver (Faraday's constant), you can calculate the total charge (and thus the total current over time) that has passed through the solution.
-
Accuracy: The voltage must be controlled carefully. Too high voltage can cause other reactions at the electrodes, like the production of oxygen or hydrogen gas, which can interfere with the accurate measurement of silver deposition.
In summary, the voltage in a silver coulometer influences the rate at which silver ions are reduced and deposited on the cathode. By controlling the voltage and measuring the mass of silver deposited, one can accurately determine the amount of electricity that has passed through the circuit.

