Composition of Water (H2O Bonds)
 Water, a seemingly simple substance, is made up of two hydrogen atoms bonded to one oxygen atom, forming the molecule H₂O. Despite its simplicity, the molecular structure of water is crucial to understanding its behavior and the potential to use it as a fuel source. The atoms in a water molecule are held together by covalent bonds, which means that the hydrogen and oxygen atoms share electrons, creating a stable molecular structure.
Water, a seemingly simple substance, is made up of two hydrogen atoms bonded to one oxygen atom, forming the molecule H₂O. Despite its simplicity, the molecular structure of water is crucial to understanding its behavior and the potential to use it as a fuel source. The atoms in a water molecule are held together by covalent bonds, which means that the hydrogen and oxygen atoms share electrons, creating a stable molecular structure.
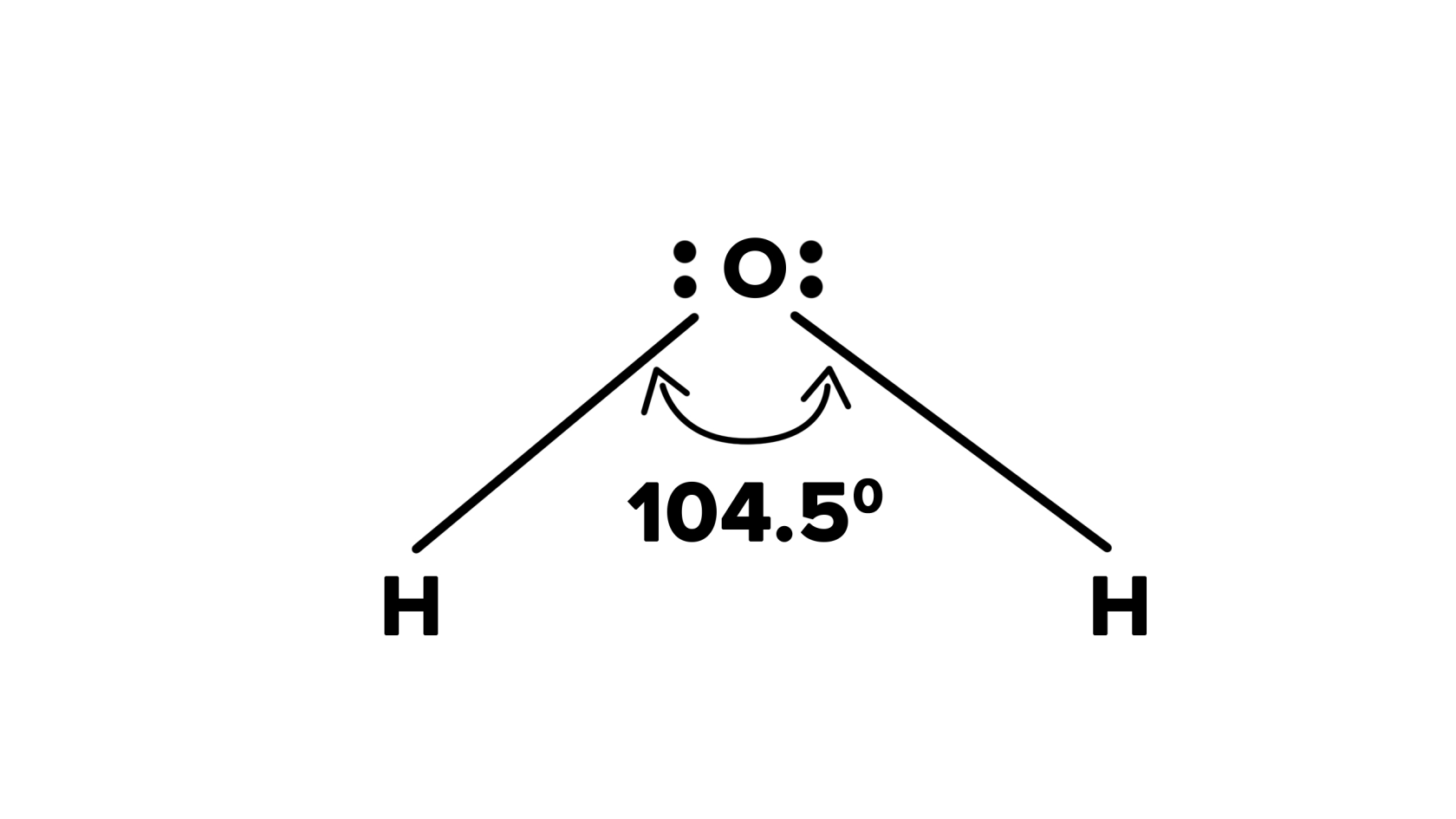
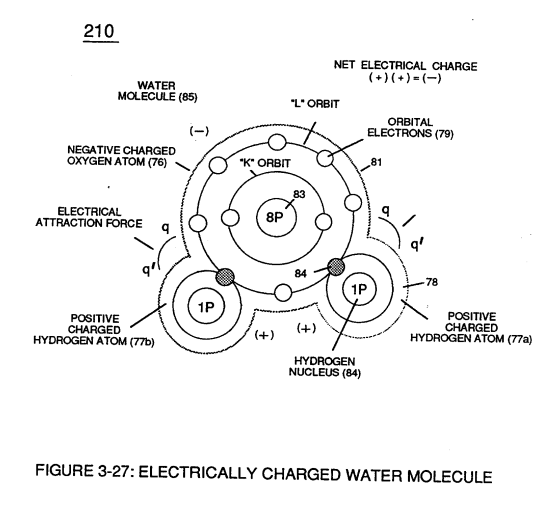
In a water molecule, the oxygen atom is much more electronegative than the hydrogen atoms, meaning it has a stronger pull on the shared electrons. This creates a polar covalent bond, with the oxygen side of the molecule having a slight negative charge and the hydrogen side having a slight positive charge. This polarity gives water many of its unique properties, such as its ability to dissolve a wide range of substances and its high surface tension.
The angle between the two hydrogen atoms in a water molecule is approximately 104.5 degrees, giving the molecule a bent shape. This molecular geometry contributes to the dipole nature of water, making it highly effective in interactions with other polar molecules and ions. The strength of the covalent bonds within the water molecule means that a significant amount of energy is required to break these bonds and separate the hydrogen and oxygen atoms.
In Stanley Meyer’s water fuel cell technology, the goal is to efficiently break these covalent bonds to release hydrogen and oxygen gases, which can then be used as fuel. Understanding the composition of water and the nature of its bonds is essential for appreciating the challenges involved in splitting water molecules and the innovative methods Meyer employed to overcome these challenges. By using high-voltage pulses and resonance, Meyer aimed to weaken these bonds in a more energy-efficient manner than traditional electrolysis, making water a viable fuel source for various applications.