How Voltage Influences Molecules
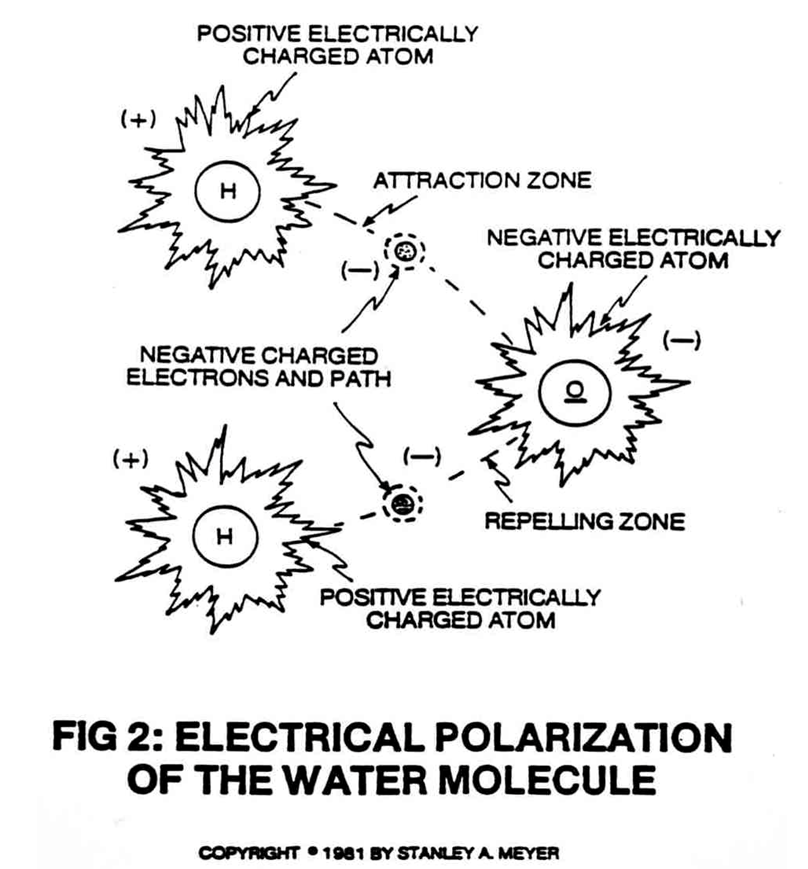 Voltage plays a crucial role in altering the behavior of molecules, particularly in the context of electrolysis and hydrogen production. In a water molecule (H₂O), the hydrogen and oxygen atoms are held together by covalent bonds. These bonds are strong, and breaking them requires energy. Voltage is one way to supply this energy to the water molecules, allowing them to dissociate into hydrogen and oxygen.
Voltage plays a crucial role in altering the behavior of molecules, particularly in the context of electrolysis and hydrogen production. In a water molecule (H₂O), the hydrogen and oxygen atoms are held together by covalent bonds. These bonds are strong, and breaking them requires energy. Voltage is one way to supply this energy to the water molecules, allowing them to dissociate into hydrogen and oxygen.
In traditional electrolysis, a direct current (DC) voltage is applied across electrodes submerged in water, creating an electric field that influences the molecules. The positive voltage applied to the anode attracts the negatively charged oxygen ions, while the negative voltage at the cathode attracts the positively charged hydrogen ions. This causes the water molecules to split, releasing hydrogen gas at the cathode and oxygen gas at the anode. The applied voltage must be sufficient to overcome the binding energy of the covalent bonds in water, which makes the process energy-intensive.
Stanley Meyer’s water fuel cell technology took a different approach to using voltage to influence water molecules. Instead of applying a constant voltage, Meyer used high-voltage pulses to stimulate the water. By using electrical resonance, Meyer sought to match the natural frequency of the water molecules, making it easier to break the bonds. The high-voltage pulses created an oscillating electric field that polarized the water molecules, weakening the bonds between the hydrogen and oxygen atoms. This polarization effect was intended to make the dissociation of water molecules more efficient, reducing the amount of energy required compared to conventional electrolysis.
Voltage also influences molecules by creating a dipole effect. In water, the oxygen atom is more electronegative than the hydrogen atoms, resulting in a polar molecule with a partial negative charge on the oxygen and partial positive charges on the hydrogens. When a voltage is applied, it enhances this polarity, aligning the water molecules in the direction of the electric field. This alignment weakens the covalent bonds, making it easier for the high-voltage pulses to split the molecules.
Meyer’s approach to voltage stimulation was based on the idea that by carefully controlling the frequency and magnitude of the applied voltage, it was possible to achieve resonance with the water molecules. This resonance would amplify the energy effect of the voltage, allowing the bonds to break with less input energy. The concept of using voltage to influence molecular behavior in this way was central to Meyer’s goal of creating an energy-efficient method for hydrogen production from water.
In summary, voltage is a key factor in determining how water molecules behave and how easily they can be split into hydrogen and oxygen. By using high-voltage pulses and resonance, Stanley Meyer aimed to make the process of splitting water molecules more efficient, potentially paving the way for a clean and sustainable energy source.
