What is passivation of stainless steel?
What is passivation, and how does passivation work? How do I passivate stainless steel parts after machining operations? These are questions commonly asked by machine shops and manufacturers of part materials such as stainless steel, titanium and tantalum.
What is passivation of stainless steel?
Passivation is a widely-used metal finishing process to prevent corrosion. In stainless steel, the passivation process uses nitric acid or citric acid to remove free iron from the surface. The chemical treatment leads to a protective oxide layer that is less likely to chemically react with air and cause corrosion. Passivated stainless steel resists rust.
Passivation prevents rust in stainless steel
What does passivated stainless steel mean?
For manufacturers, the industry standards ASTM A967 and AMS 2700 represent the most widely used standards for passivating stainless steel. According to ASTM A967, the definition of passivation is:
the chemical treatment of stainless steel with a mild oxidant, such as a nitric acid solution, for the purpose of the removal of free iron or other foreign matter.”
Further, ASTM A380 states that passivation is:
removal of exogenous iron or iron compounds from the surface of a stainless steel by means of a chemical dissolution, most typically by a treatment with an acid solution that will remove the surface contamination but will not significantly affect the stainless steel itself … for the purpose of enhancing the spontaneous formation of the protective passive film.”
History of Passivation Process
In the mid 1800s, chemist Christian Friedrich Schönbein discovered the effect of passivation. After dipping iron in concentrated nitric acid, he found that the iron had little or no chemical reactivity compared to iron that did not receive the concentrated nitric acid treatment. His name for that lack of chemical reactivity was the “passive” condition.
As passivation of stainless steel with nitric acid became a widespread practice in the 1900s, environmental and safety issues with nitric acid became more apparent. Research done by the Adolf Coors brewing company in Germany identified citric acid as an effective alternative. In the 1990s, many manufacturers began to adopt citric acid as a safer and more environmentally friendly alternative to nitric acid.
Today the industry standards for surface passivation offer methods for nitric acid or citric acid, or nitric acid with sodium dichromate. Choice of method often depends on customer requirements. Each method has its own advantages and disadvantages. For details, please see our article Nitric vs. Citric Acid Passivation.
Why passivate stainless steel?
Passivation is a post-fabrication best practice for newly-machined stainless steel parts and components. Benefits include:
- Chemical film barrier against rust
- Extended life of the product
- Removal of contamination from product surface
- Reduced need for maintenance.
How does passivation work?
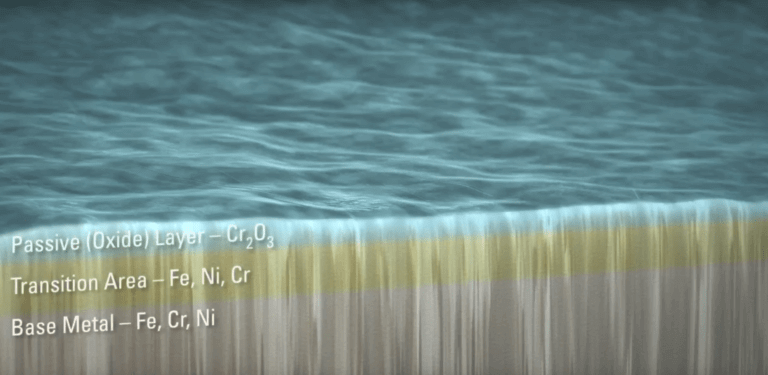
Stainless steel is an iron-based alloy, typically composed of iron, nickel and chromium. Stainless steel derives its corrosion-resistant properties from the chromium content. Chromium, when exposed to oxygen (air), forms a thin film of chromium oxide that covers the stainless steel surface and protects the underlying iron from rusting. The purpose of passivation is to augment and optimize formation of the chromium oxide layer.
Immersion of stainless steel in an acid bath dissolves free iron from the surface while leaving the chromium intact. The acid chemically removes the free iron, leaving behind a uniform surface with a higher proportion of chromium than the underlying material.
Upon exposure to oxygen in the air after the acid bath, the stainless steel forms the chromic oxide layer over the next 24 to 48 hours. The higher proportion of chromium at the surface allows for the formation of a thicker, more protective chromium oxide layer. Removal of free iron from the surface removes opportunities for corrosion to start.
The resulting passive layer provides a chemically non-reactive surface that protects against rust.
Passivation Layer on Stainless Steel
Passivation Layer – Microscopic View. Source: Astro Pak. Used with permission.
When is passivation of stainless steel required?
Passivation is a post-fabrication process that is performed after grinding, welding, cutting and other machining operations that manipulate stainless steel. Under ideal conditions, stainless steel naturally resists corrosion, which might suggest that passivating would be unnecessary.
Under normal, realistic conditions, however, any of the following can inhibit the formation of the oxide film that protects against corrosion:
- foreign material in a manufacturing environment (shop dirt, grinding swarf)
- sulfides added to the stainless steel for improved machinability
- particles of iron from cutting tools embedded in the surface of stainless steel parts.
Such contaminants must be removed down to the surface grain boundaries to restore a uniformly corrosion-resistant surface. The passivation process corrects these issues.
What passivation is NOT
- Not electrolytic. Passivation is a chemical treatment and not an electrolytic process. Passivation does not depend on electrochemical reactions, unlike electropolishing or anodizing.
- Not for scale removal. Passivating is not a method to remove oxide scale from machined parts after heat treating or welding.
- Not a coat of paint. Passivating stainless steel does not change the color or surface appearance of the metal. Passivating is not necessary for items that will be painted or powder coated.
How to passivate stainless steel
Many passivation specifications (ASTM A967, AMS 2700) exist to instruct on the proper process to passivate stainless steel, titanium and other materials. The following chemical cleaning and passivation procedure phases are common to nearly all the specifications:
- Clean – Remove any contaminants from the surface, such as grease and oils.
- Passivate – Perform chemical treatment via immersion in an acid bath, typically nitric acid or citric acid.
- Test – Test the newly passivated stainless steel surface to ensure effectiveness of the process steps.
Some stainless steel passivation specifications call for adding sodium dichromate to the nitric acid bath to provide more rapid formation of the oxide layer or passivation film. Sodium dichromate, however, is a highly toxic hexavalent chromium compound. Alternative practices include use of ultrasonic machines and citric acid such as CitriSurf® to encourage oxygen formation at the metal surface while the material is still immersed in the acid bath.
Length of time of immersion in the acid tank is typically 20 – 30 minutes. Temperature specifications for the acid can vary, depending on the grade of stainless steel and the acid chemistry, but typically fall between 120 – 150 °F.
Video: Fully Automated Citric Acid Passivation System
Process steps for passivating stainless steel parts
Putting together a passivation line requires a process that will both clean and passivate stainless steel. Common process steps for passivating stainless steel are as follows:
- Alkaline cleaning of the materials to remove all contaminants, oils, and foreign materials. Commonly uses detergent cleaners like sodium hydroxide, Micro-90, or Simple Green.
- Water rinse – Commonly with DI (Deionized) water or RO (Reverse Osmosis) Water in high-precision industries
- Nitric acid or citric acid (CitriSurf) immersion bath to fully dissolve any free irons and sulfides and expedite the formation of passive film or oxide layer
- Water rinse – Commonly with DI water in high-precision industries
- Second water rinse – Commonly with DI Water in high-precision industries
- Dry parts
- Test sample parts via specification standards using: salt spray, high humidity chamber exposure, or copper sulfate testing.
What to watch for with passivation of metals
Passivation can be thought of as controlled corrosion. The acid bath dissolves, or corrodes, free iron at the surface in a uniform, controlled manner. When not controlled properly, runaway corrosion can occur in a phenomenon known as “flash attack.” In flash attack, the metal develops a dark, heavily etched surface – exactly the corrosion that the passive layer is intended to prevent.
Keeping the acid solution free of contaminants is critical for preventing flash attack. Often the remedy is as simple as refilling the acid bath with fresh solution. Replacing the acid solution on a regular schedule is recommended to prevent build-up of contaminants in the solution. Use of a higher grade of water (RO water or DI water) with fewer chlorides than tap water may also resolve issues with flash attack.
Thorough cleaning of stainless steel parts BEFORE the acid bath is also crucial. Any grease or cutting oil left on the parts tends to form bubbles that interfere with the process. In these cases, consider using a degreaser or changing detergents to ensure that the part is completely free of contaminants. In some cases thermal oxides from heat treating or welding may require grinding or pickling for removal before passivating.
Avoid mixing grades of stainless steel (e.g. 300 series and 400 series) in the acid bath at the same time, as this risks galvanic corrosion. In this situation, the less noble metal corrodes faster than it would have if the dissimilar metals had not been in contact in the solution.
What passivation equipment do I need?
Best Technology is recognized as an industry leader in passivation equipment, tanks, systems and lines. Our experts understand the careful balance of chemistry, temperature and immersion time to meet passivation specifications and to avoid costly errors. We offer a broad range of equipment from tabletop machines to integrated wet benches to fully automated systems. Our application engineers can design equipment to meet your requirements and specifications.
As you gather information on starting a new passivation line, be sure to check out our passivation process checklist. When you’re ready, contact us to talk with our passivation process experts.
Types of passivation equipment
Passivation equipment is available in a variety of tank sizes. The smallest systems start with a tank size of 1.25 gallon, while the largest systems run to 500+ gallons. A passivation system offers the integrated convenience of facilitating multiple process steps (e.g., wash, rinse, passivate, rinse and dry) in a single unified piece of equipment.
Types of systems include:
- Small benchtop passivation equipment
- Wet bench passivation equipment
- Automated passivation systems
- Agitated immersion passivation systems
Click any of the following images to learn more about that type of equipment.
Small Benchtop Passivation Equipment
Wet Bench Passivation Equipment
Agitated Immersion Passivation Systems
Passivation standards and specifications
In the aerospace and medical device industries, many high-precision manufacturers face additional guidelines, specifications, regulations and accreditation standards when passivating their products. One such accreditation is NADCAP, or National Aerospace and Defense Contractors Accreditation Program. Use of an automated passivation system ensures tight, documented process control parameters to meet validation requirements.
Process Validation FAQs
Industry Standards – Passivation Specifications
Looking for a stainless steel passivation specification? Numerous industry standards exist to provide a “how-to-passivate” definition. The most commonly used standards are ASTM A967 and AMS 2700.
| Standard | Title / Description |
| ASTM A967 |
Standard Specification for Chemical Passivation Treatments for Stainless Steel Parts
|
| AMS 2700 |
Passivation of Corrosion Resistant Steels
|
| ASTM A380 |
Standard Practice for Cleaning, Descaling, and Passivation of Stainless Steel Parts, Equipment, and Systems
|
| AMS-QQ-P-35 |
(superseded) Passivation Treatments for Corrosion-Resistant Steel
|
| ASTM F86 | Standard Practice for Surface Preparation and Marking of Metallic Surgical Implants |
| ASTM F983 | Standard Practice for Permanent Marking of Orthopaedic Implant Components |
| ASTM B600 |
Standard Guide for Descaling and Cleaning Titanium and Titanium Alloy Surfaces
|
| AMS-STD-753 | Corrosion-Resistant Steel Parts: Sampling, Inspection and Testing for Surface Passivation |
| BS (British Standard) EN 2516 | Aerospace Series: Passivation of Corrosion Resisting Steels and Decontamination of Nickel Base Alloys |
Military Specs and Standards
| Standard | Refers to | Title / Description |
| MIL-HDBK-808 | QQ-P-35 MIL-STD-753 |
Finish, Protective and Codes for Finishing Schemes for Ground and Ground Support Equipment:
|
| MIL-DTL-14072 | ASTM A380 |
Finishes for Ground Based Electronic Equipment:
|
| MIL-DTL-5002 | ASTM A967 AMS 2700 |
Surface Treatments and Inorganic Coatings For Metal Surfaces of Weapons Systems:
|
| MIL-STD-171 | ASTM A967 AMS 2700 ASTM A380 |
Finishing of Metal and Wood Surfaces:
|
Industry-Leading Expertise
Aerospace and medical device manufacturers depend on Best Technology’s expertise for equipment and process design and development. Inquire today for more information on how your company can benefit from our passivation equipment and process design.