Appendix 3
RE: Table of Tabulation
Appendix A
(Tab 35)
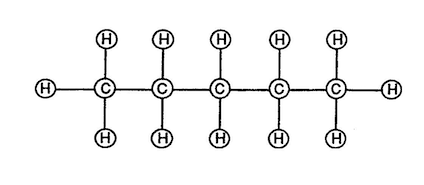
Natural Gas is composed of (5) carbon atoms and (12) hydrogen atoms to form a molecule of gas.
Atomic Mass Unit:
1 Electron (E) = 1 Proton (P) ... 1 Mu
Hydrogen Atom: 1 E = 1P ... 1Mu
Carbon Atom: 6 E = 6P ... 6 Mu
Atomic Mass Ratio (Mur) of Natural Gas:
(12H x 1 Mu) plus (5C x 6 Mu) = 42 Mu's
See Appendix (B) Note (2)
Whereby
12H (Mu) divided by 42 (Mu's) = 28% of gas pound (lb).
Thus,
One pound (lb) of Natural Gas contains .28 lb of Hydrogen Atoms.
Molecular Structure Of Natural Gas
(Volumetric Displacement of Atom spheres)
Fuel Gas Contaminates: Cryogenic Processing:
12% Non-burnable Contaminates (carbon dioxide, heavy hydrocarbons, and water vapor)
.28 lbs of hydrogen atoms x 12% = .28 lbs - .033 = .247 lbs Hydrogen atoms
Energy-Yield Potential:
.247 lbs hydrogen atoms - 10% (absorption Contaminates) = .247 - .024 = .223 lbs of hydrogen atoms available for gas combustion per pound of Natural Gas approximately.
Thereby
As to Normal Gas Burning Levels, One pound (1 lb) of water contains approx. (.185) lbs of Hydrogen Atoms as compared to One pound (1 lb) of Natural Gas which contains approx. (.223) lbs of Hydrogen Atoms. Water, of course, supplies its own oxygen to support the combustion process; whereas, Natural Gas must extract oxygen from air to produce thermal heat.
Energy Enhancement Process:
Energy Yield Enhancement of water is increased beyond Natural Gas burning rate by way of the Hydrogen Fracturing Process which simply prevents and/or retards the formation of the water molecule during thermal gas ignition/combustion ... Energy priming the combustible gas atoms by stimulating the Atomic Energy Balance of Water (memo WFC 424) undergoing "Voltage Tickling of State Space" ... to cause "Particle Oscillation" as a "Energy Generator".
Stanley A. Meyer
Appx. A 03