Appendix A
RE: Table of Tabulation
Appendix A
(Tab 34)
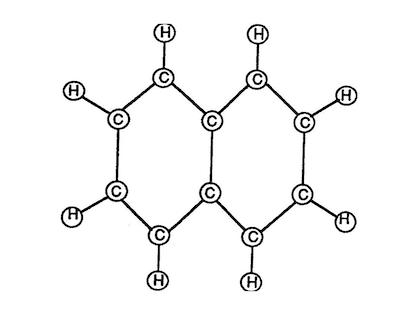
Gasoline is composed of (2) Carbon atoms and (8) Hydrogen atoms to form a gasoline molecule.
Atomic Mass Unit:
I Electron = 1 Proton - 1 Mu Hydrogen
Atom: IE = 1P - 1 Mu Carbon Atom:
6E = 6P - 6 Mu Atomic Mass Ratio (Mur) of Gasoline:
(8 H X 1Mu) plus (1 Oxy 6Mu) = 68 Mu's
See Appendix (B) Note (2)
Whereby,
8H (Mu) divided by 68 (Mu's) = 11.7% Hydrogen Atoms
Thus,
One gallon of Gasoline equals 5.61 lbs/gal.
5.61 lbs/gal. times .117 = 0.6561 lbs of Hydrogen / Gasoline gal.
Molecular Structure of Gasoline
(Volumetric Displacement of Atom Spheres)
Fuel-contaminates: Distillation performance Point
Chromatogram of typical Gasoline:
degree C = (degree F - 32) /1.8 @ 437 degrees F. ..... 10% / Volume impurities (Vi)
Therefore
.656 lbs of Hydrogen / Gasoline - .065 (Vi) = .5911 lbs of Hydrogen Atoms available for Gas Combustion per gallon of Gasoline approximately.
Thermal Heat of Combustion
Water / gallon ...........57,000 BTU'S approx.
Gasoline / gallon ........ 22,800 BTU'S approx.
Thereby
Water Energy-yield (By) is 2.5 times greater than Gasoline since the hydrogen content of water is more than twice that of fossil fuel of gasoline. (See U.S. National Bureau of Standards Monograph 168 (523 pages )(Feb.198 I ) Engineering Design Data Manual titled "Selected Properties of Hydrogen", CODEN NBSM A6 Library of Congress Catalog Card Number: 80-6(0195)).
Stanley A. Meyer
Appx. A 02