Application Notes
RE: Table of Tabulation
Appendix A
(Tab 33)
Application Notes
Water vs. Fossil-Fuel Energy Content
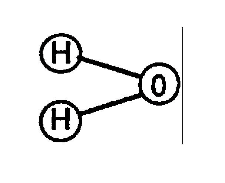
Water is composed of (2) Hydrogen Atoms and (1) Oxygen Atom to form a molecule of Water.
Atomic Mass Unit:
Electron (E) = 1 Proton (P) - 1Mu Hydrogen
Atom: IE = IP - IMu Oxygen Atom: 8E = 8P - 8Mu Atomic Mass Ratio (Mur) of Water
(2H X 1Mu) plus (1 Oxy. X 8 Mu) = 10 Mu's
See Appendix (B) Note (2)
Whereby,
2H (Mu) divided by (10 Mu's) = 20%
Thus,
One gallon of Water contains 1.6691 lbs. of Hydrogen
Molecular Structure of Water
(Volumetric Displacement of Atom spheres)
Energy-Yield Potential of Water
One water gallon equals 8.345 lbs
8.345 lbs x .20 = 1.669 pounds of Hydrogen / H₂O gal.
1.669 pounds of hydrogen-fuel of water - .183591 lbs (11% per volume of impurities ...
typically 20 ppm - 40 ppm contaminates with Ambient Air being present) =
1.4854 lbs of hydrogen atoms available for gas combustion per gallon of Water approximately.
Water as Fuel
The by-product of burning gases derived from Water is environmentally safe since there is no .UOOJIS present in the Water molecule ... resulting in the re-formation of Water "mist" after gas combustion... being able to re-energize the newly formed Water Droplets for energy "reuse" once exposed to Sunlight. (See Energy recycling graph 530 of Figure 5-6, once again)
Stanley A. Meyer
Appx. A 01