ATOMIC INTERACTION TO VOLTAGE STIMULATION
Atomic structure of an atom exhibits two types of electrical charged mass-entities, orbital electrons having negative electrical charges (-) and a Nucleus (at least one proton) having a positive electrical charge (+).
The positive electrical charge of the Nucleus equals the sum total of all negative electrical charged electrons when the Atom is in “stable-state.”
In stable-state or normal-state, the number of electrons equals the number of protons to give the atom “NO” net electrical charge.
Whenever one or more electrons are “dislodged” from the atom, the atom takes-on a net positive electrical charge and is called a positive ion.
If an electron combines with an stable or normal atom, the atom has a net negative charge and is called a negative ion.
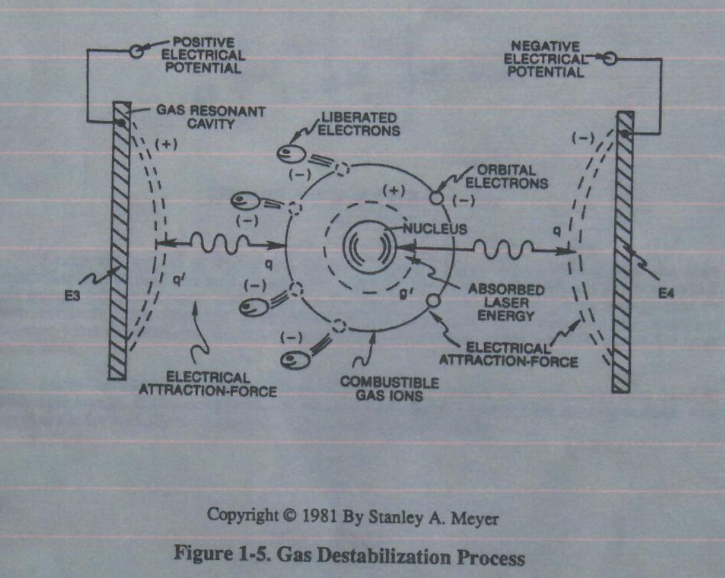
Voltage potential within an electrical circuit can cause one or more electrons to be dislodged from the atom due to opposite electrical polarity attraction between unlike charged entities, as shown in Figure 1-5 (see Figure 1-3 again) as to Newton’s and Coulomb’s Laws of electrical-force.
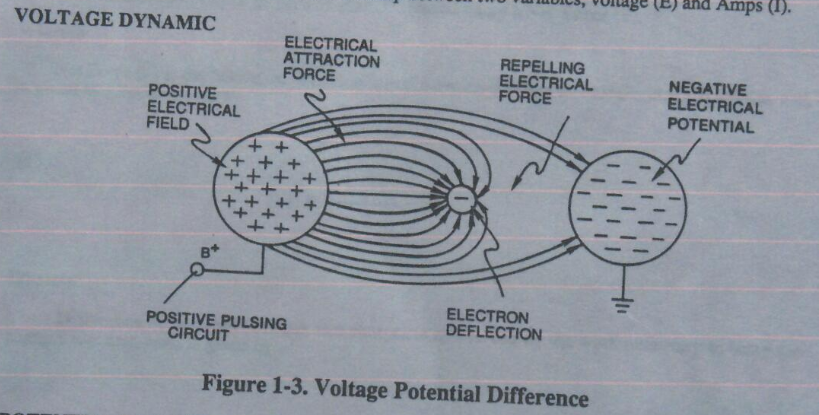 |
 Newton’s and Coulomb’s Laws of electrical-force is used to combine or join atoms together by way of Covalent Bonding (opposite electrical forces) to form liquids, solids, or air.
Newton’s and Coulomb’s Laws of electrical-force is used to combine or join atoms together by way of Covalent Bonding (opposite electrical forces) to form liquids, solids, or air.
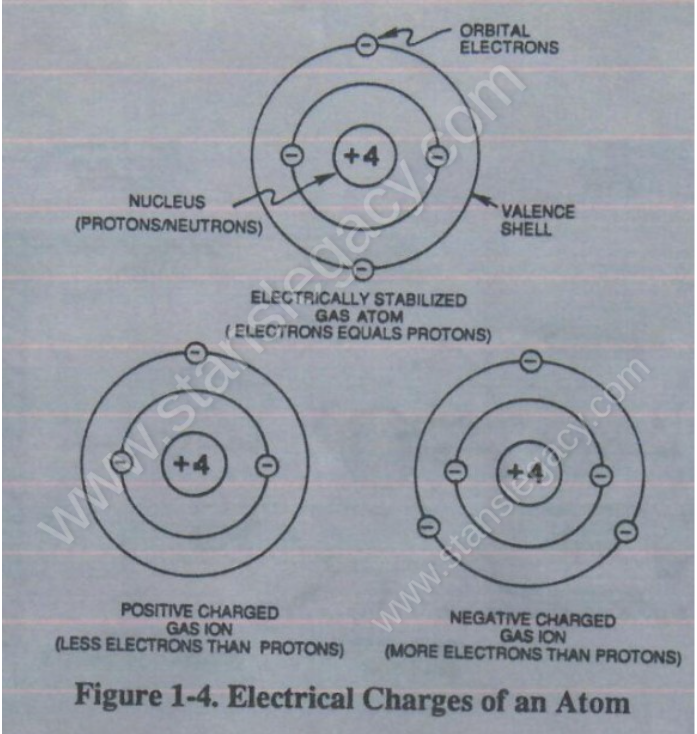
The atom of Argon Gas (Ar) is, naturally, electrically stabilized when the number of electrons around the number of protons equal the atomic number of 18 on the Periodic Table of Elements.
The illustrated Argon Atom becomes electrical charged ion when the electron number changes from 18, as illustrated in Figure 1-4.