Atomic Interaction to Voltage Stimulation
Atomic structure of an atom exhibits two types of electrical charged mass-entities.
Orbital electrons having negative electrical charges (-) and a nucleus composed of protons having positive electrical charges (+).
In stable electrical state, the number of negative electrically charged electrons equals the same number of positive electrically charged protons ... forming an atom having "no" net electrical charge.
Whenever one or more electrons are "dislodged" from the atom, the atom takes on a net positive electrical charge and is called a positive ion.
If an electron combines with a stable or normal atom, the atom has a net negative charge and is called a negative ion.
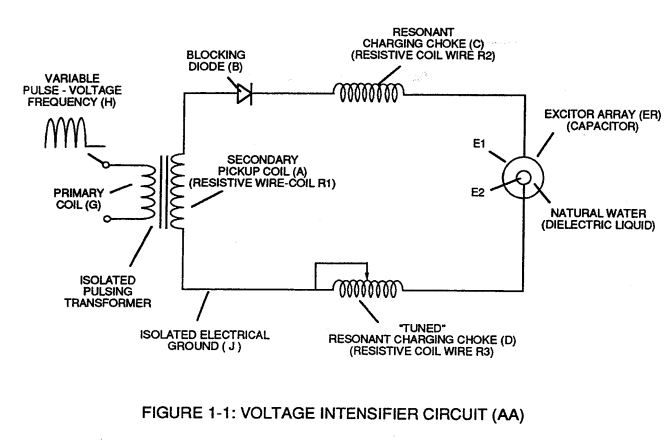
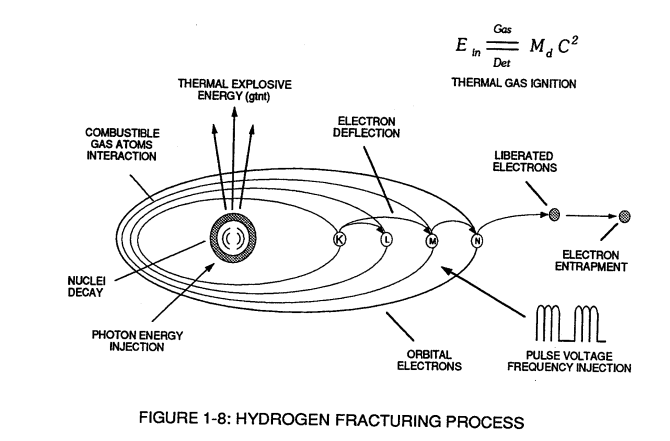
Voltage potential within an electrical circuit (see Voltage Intensifier Circuit as to Figure 1-1) can cause one or more electrons to be dislodged from the atom due to opposite polarity attraction between unlike charged entities, as shown in Figure (1-8).
|
Voltage Intensifier Circuit as to Figure 1-1 |
Figure (1-8)
|
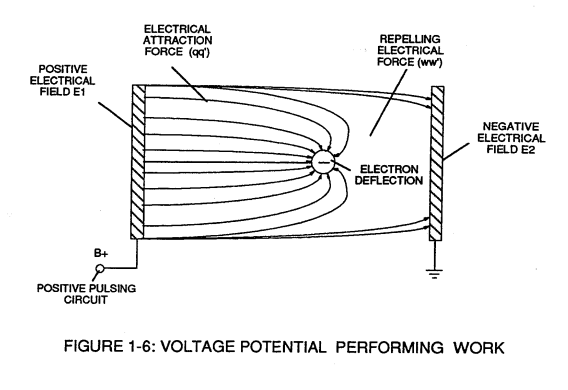
(see Figure 1-6 again as to Figure 1-9) as to Newton's and Coulomb's Laws of electrical force (RR).
The resultant electrical attraction force (qq') combines or joins unlike atoms together by way of covalent bonding to form molecules of gases, solids, or liquids.
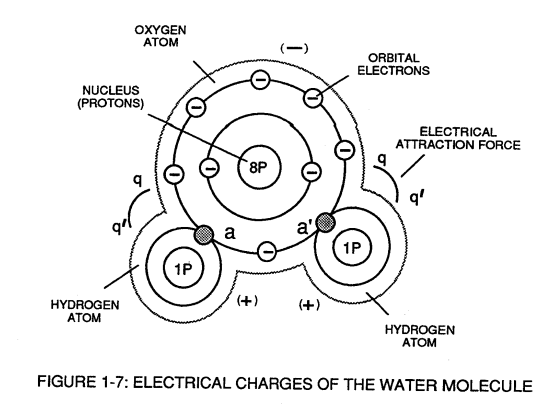 When the unlike oxygen atom combines with two hydrogen atoms to from the water molecule by accepting the hydrogen electrons (aa' of Figure 1-7), the oxygen atoms become "net" negative electrically charged (-) since the restructured oxygen atom now occupies 10 negative electrically charged electrons as to only 8 positive electrically charged protons.
When the unlike oxygen atom combines with two hydrogen atoms to from the water molecule by accepting the hydrogen electrons (aa' of Figure 1-7), the oxygen atoms become "net" negative electrically charged (-) since the restructured oxygen atom now occupies 10 negative electrically charged electrons as to only 8 positive electrically charged protons.
The hydrogen atom with only its positive charged proton remaining and unused, now, takes on a "net" positive electrical charge equal to the electrical intensity of the negative charges of the two electrons (aa') being shared by the oxygen atom.
... satisfying the law of physics that for every action there is an equal and opposite reaction.
The sum total of the two positive charged hydrogen atoms (++) equaling the negative charged oxygen atom (--) forms a "no" net electrical charged molecule of water.
Only the unlike atoms of the water molecule exhibits opposite electrical charges.