Magnetic Gas Lattice
 The forming Argon ion (Ar+) is now exposed to Iron ions (Fe+) (magnetic properties) experiencing and undergoing the same Electron Extraction Process.
The forming Argon ion (Ar+) is now exposed to Iron ions (Fe+) (magnetic properties) experiencing and undergoing the same Electron Extraction Process.
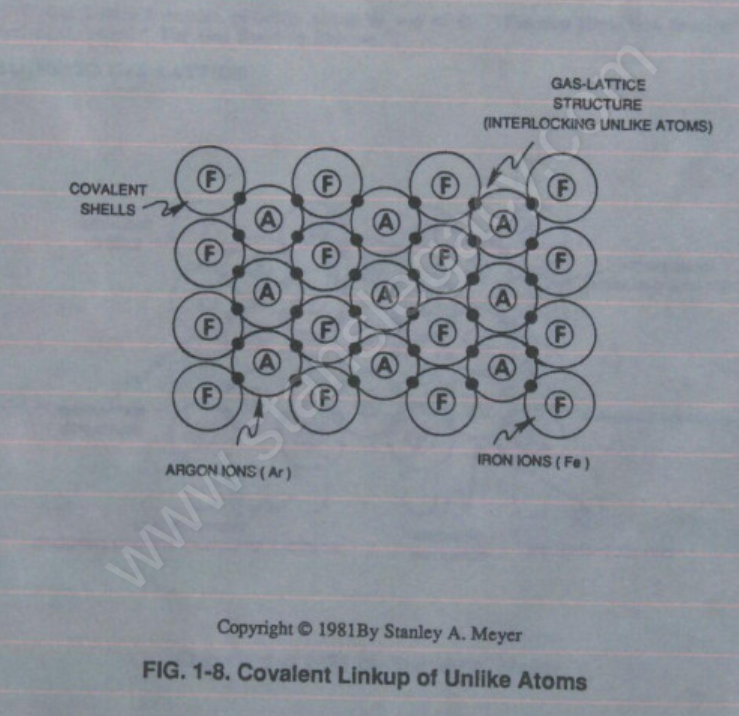
Together, the two ions (Ar+/Fe+) form a covalent link up or covalent bond when the covalent electron of the Argon ion (Ar+) pair up and be shared with the valence electron of the Iron ion (Fe+).
Covalent bonding of Iron ions (Fe+) to the Argon ion (Ar+) continues until a geometrical Gas-Lattice Structure is formed, as illustrated in Figure 1-8.
Stable-state of the Gas-Lattice occurs when the covalent shell of each unlike atom structure becomes full or filled up… the Argon atom (Ar) sees an covalent shell of 8 electrons while, at the same time, the Iron atom (Fe) sees an covalent shell (M shell) of 14 electrons.
Covalent bonding between like atoms does not occur due to the “stronger” Electrical Attraction-Force (qq') between the unlike atoms.
During Gas-Lattice formation, Iron ions (Fe+) can be replaced by other atoms exhibiting magnetic properties such as Nickel ions (Ni+) or Cobalt ions (Co+).
Gas-Lattice formation of unlike atoms by way of the Electron Extraction Process is, hereinafter, called "The Gas Bonding Process".
