Hydrogen Fracturing Process
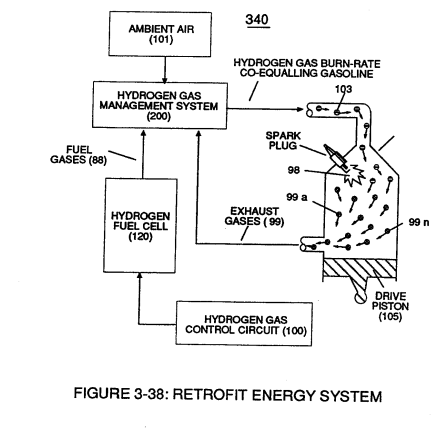 Incoming processed hydrogen fuel gas (103) is, now, exposed to thermal spark ignition process (98) which triggers thermal explosive energy-yield (gtnt) (127) that causes piston-action (105) of Figure (3-38) to exceed normal gas combustion process associated with hydrogen to air mixture of gases in stable state.
Incoming processed hydrogen fuel gas (103) is, now, exposed to thermal spark ignition process (98) which triggers thermal explosive energy-yield (gtnt) (127) that causes piston-action (105) of Figure (3-38) to exceed normal gas combustion process associated with hydrogen to air mixture of gases in stable state.
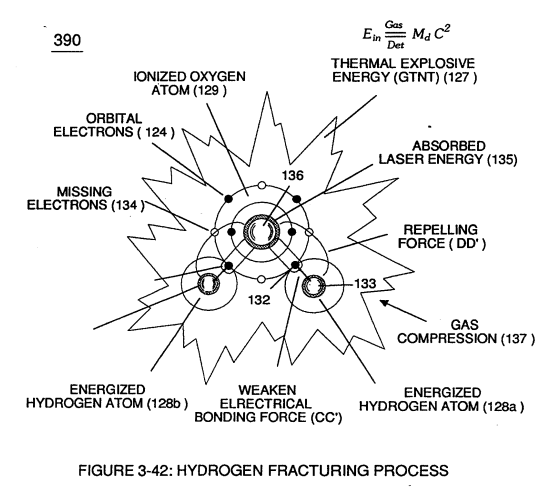
Thermal atomic interaction (127) is caused when sub-critical gas ions (104a xxx 104n) (derived from both water bath ~ and ambient air gases) fails to unite with or covalently link up or covalent bond with highly energized (laser primed) hydrogen atom (128).
Sub-critical Oxygen atom (129) having less than the normal amount of covalent electrons (orbital electrons) is unable to reach "stable-state" (six to eight covalent electrons required) when the two hydrogen atoms (128 a/b) seek to form the water molecule during thermal gas ignition.
Absorbed laser energy (131) of hydrogen gas atom nucleus (133) weakens "electrical bonding" force (CC') between hydrogen atom electron (132) and hydrogen atom nucleus (133);
while, at the same time, absorbed laser energy (135) prevents oxygen atom (129) from reaching "stable state" when electrical attraction force (BB') (opposite electrical attraction force being equivalent to the number of missing electrons) locks onto and pulls away hydrogen atom electron (132) while repelling force (DD') keeps the two positive charged nucleus's (133/136) apart.
 These "'abnormal" and "unstable" conditions coupled with thermal interaction (gas ignition) under gas compression (137) of Figure (3-42) as to Figure (3-38) (fuel-gas 88 being compressed via piston-action 105) causes combustible gas atoms (129 and 128a/b) to decay ... releasing thermal explosive energy (gtnt) (127) under control means.
These "'abnormal" and "unstable" conditions coupled with thermal interaction (gas ignition) under gas compression (137) of Figure (3-42) as to Figure (3-38) (fuel-gas 88 being compressed via piston-action 105) causes combustible gas atoms (129 and 128a/b) to decay ... releasing thermal explosive energy (gtnt) (127) under control means.
This atomic thermal-interaction between sub-critical combustible gas atoms (127 and 128a/b) is, now, herein after called "The Hydrogen fracturing Process. "
