Electrovalent Bonding
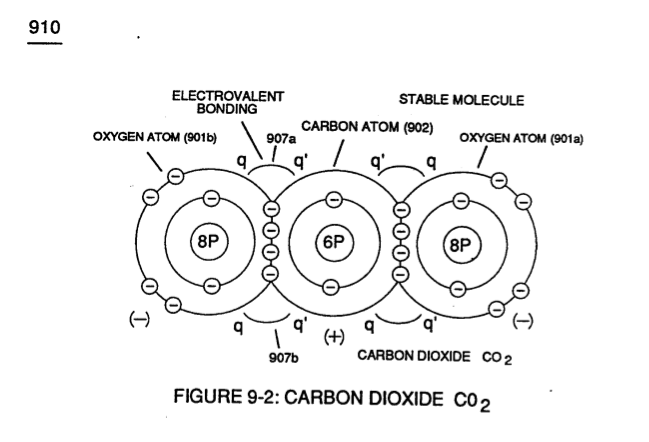
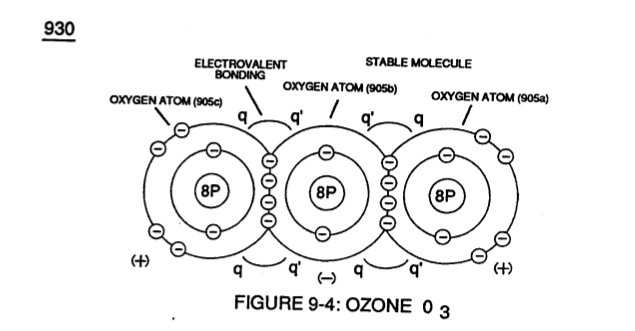
In similar manner by which polar Water Molecule unlike atoms (Hydrogen Atoms 78 / Oxygen Atom ID (210) of Figure (3-27) take-on opposite electrical Charges (B+ / B-), other gas-atoms molecule (s) experience the same Electrical Charge Effect (q - q') when covalent-electron sharing occurs, as illustrated in polar-molecule Carbon Dioxide CO2 (910) of Figure (9-2) as to allotropic molecule of Ozone 03 (930) of Figure (9-4).
(210) of Figure (3-27)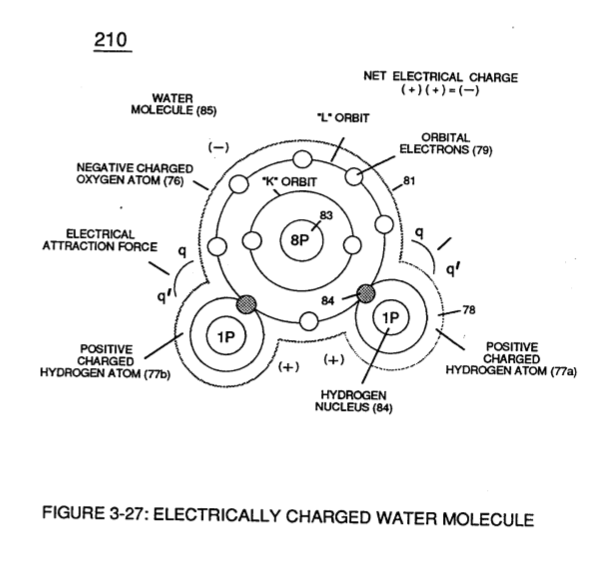
|
(910) of Figure (9-2) |
(930) of Figure (9-4) |
Carbon Dioxide molecule 0>2 (910) Electrovalent Bonding forces (q - q') comes into existence when unlike Carbon Atom (902) shares electrons with each of two Oxygen Atoms (901a / 901b) since the accepted and captured covalent electrons migrates toward both oxygen atom (901a and 901b) nucleus proton-cluster of eight particles having a greater total positive static charge than Carbon Atom (902) nucleus proton-cluster of only six ... forming polar charged (B+ / B- ) Carbon Dioxide CO2 molecule (910).
The additive two captured/accepted electrons (total ten 10 electrons as to only eight B protons) causes both oxygen atoms (901a / 90lb) to individually take-on a negative electrical charge (B-) while the center positioned Carbon Atom (902) emanates a positive electrical charge (B+) of equal but opposite electrical intensity (q - q') when its shared electrons is/are being used/accepted by unlike oxygen atoms (901a / 901b).
N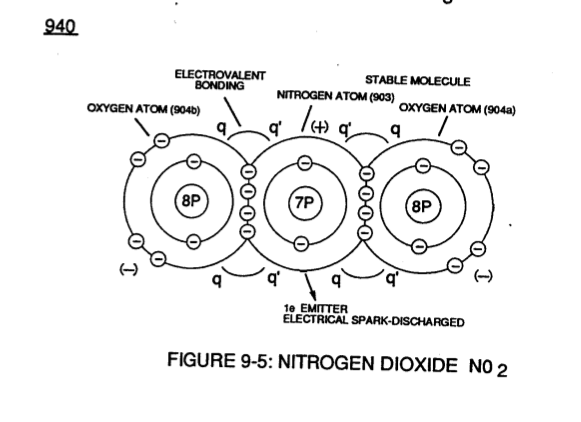 itrogen Dioxide N02 (940) of Figure (9-5) is another example of polar electrical charging (q _ q') of two unlike atoms forming a stable molecule wherein a Nitrogen Atom (N) (903) covalently interlocks with two Oxygen Atoms (904a / 904b).
itrogen Dioxide N02 (940) of Figure (9-5) is another example of polar electrical charging (q _ q') of two unlike atoms forming a stable molecule wherein a Nitrogen Atom (N) (903) covalently interlocks with two Oxygen Atoms (904a / 904b).
Identical gas-atoms of Oxygen Atoms (905a / 905b 1905c) of Figure (9-4) further exhibits Electrical Charging Effect (q - q') since in all cases the second Electron Shell (L-orbit) can accept up to eight (8) electrons to cause molecule stabilization.
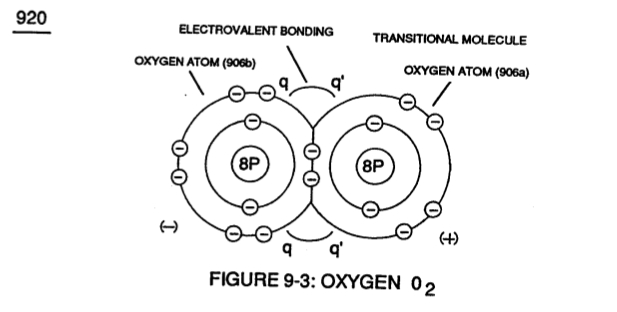 Transitional gas-molecule of Oxygen 02 combines together two oxygen atoms (906a / 906b) in this way while allowing the donor oxygen atom (906b) to except another oxygen atom (905c) of Figure (9-4) since it's L-Orbit (Outer Shell) still has available two "Electron-Gaps" for covalent Linkup, as illustrated in (920) of Figure (9-3).
Transitional gas-molecule of Oxygen 02 combines together two oxygen atoms (906a / 906b) in this way while allowing the donor oxygen atom (906b) to except another oxygen atom (905c) of Figure (9-4) since it's L-Orbit (Outer Shell) still has available two "Electron-Gaps" for covalent Linkup, as illustrated in (920) of Figure (9-3).
Electrical Charging Effect (q -g') is Electrical Attraction Force (q - q') of opposite electrical polarity between the established positive (B+) electrically charged atom (s) and the negative (B-) electrical charged atom (s).
Electrical intensity of Opposite Electrical Attraction Force (q-qa'- 907a + q-qb' - 907b) (herein after called Electrovalent Bonding) (total electrical bonding force between two opposite electrical charged atoms) are equivalent to the total number of electrons being used/accepted by the host atom (s) having the greater positive charged (B+) nucleus as so established under the laws of physics which states for "every action there is an equal and opposite reaction".
This is possible due the fact that all orbiting individual electrons display their own negative electrical charge (B-) whereas each proton-particle separately supports a positive electrical charge (B+)
... both opposite electrical charged particles (Proton as to each Electron) being equal in electrical magnitude (B+ / B-).
And due to the fact that the oxygen atom does not take-on an electromagnetic charged field since its electrons pair together and spin in opposite direction.