Electrically Charged Water Molecule
 Atomic structure of an atom (76) and (77) of Figure (3-27) exhibits two types of electrical charged mass entities, orbital electrons (79) having negative electrical charges ( - ) and a nucleus (84) (at least one proton) having a positive electrical charge ( + ).
Atomic structure of an atom (76) and (77) of Figure (3-27) exhibits two types of electrical charged mass entities, orbital electrons (79) having negative electrical charges ( - ) and a nucleus (84) (at least one proton) having a positive electrical charge ( + ).
The positive electrical charge of the nucleus equals the sum total of all negative electrical charged electrons when the atom is in "stable-state."
In stable state or normal-state, the number of electrons equals the number of protons to give the atom "no" net electrical charge.
Whenever one or more electrons are "dislodged" from the atom, the atom takes-on a net positive electrical charge and is called a positive ion.
If a electron combines with a stable or normal atom, the atom has a net negative charge and is called a negative ion.
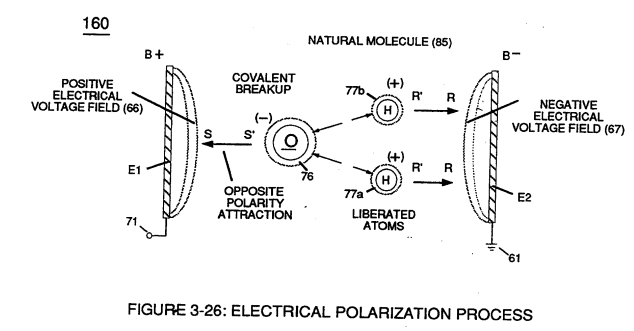
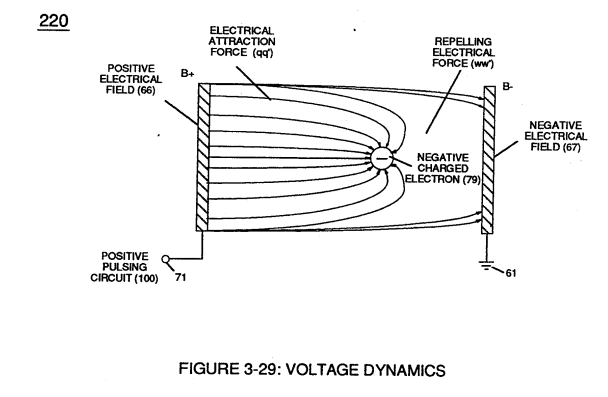
Voltage potential (65) within electrical circuit (60) can cause one or more electrons (79) to be dislodged from the water molecule atom (85) of Figure (3-26) due to opposite electrical polarity attraction (qq') of Figure (3-29) between unlike charged entities, as shown in (160) of Figure (326) as to Newton's and Coulomb's laws of electrical-force.
|
Figure (3-26) |
Figure (3-29) |
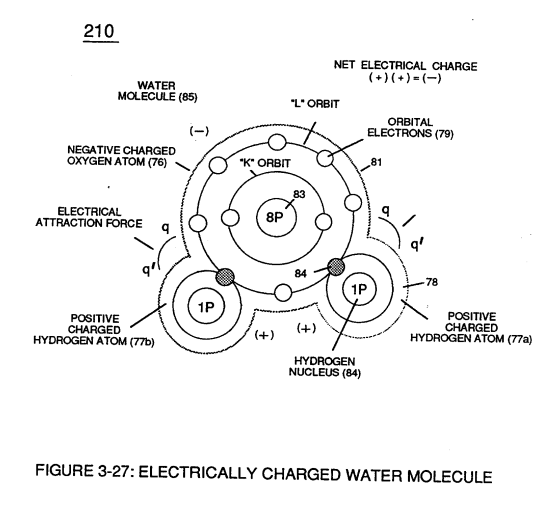
These same laws of electrical-force (qq') are used to combine or join atoms together by way of covalent bonding (opposite electrical forces) to form a molecule of water (85), as illustrated in (210) of Figure (3-27).
The liquid molecule of water (210) of Figure (3-27) is formed when the two hydrogen atoms (77a1b) takes-on a net "positive electrical charge" (78), which is, equal to the net "negative electrical charge" (81) of the oxygen atom (76).
The resultant electrical force (qq') between the opposite electrical charged hydrogen (77) and oxygen (76) atoms keeps water molecule (210) intact when the hydrogen atom (77) shares its electron (84) with oxygen atom (76).
The electrical strength of attraction force (qq') between the water molecule atoms is determined by the electrical size of the hydrogen atoms and the displacement of its negative charged electrons (84) during covalent sharing.
 Oxygen atom becomes negative electrical charged (81) since oxygen atom (76), now, has a total of ten negative charged electrons (79a xxx 79n) in its "K" plus "L" orbits while maintaining it's original eight positive charged protons which makes up oxygen nucleus (83).
Oxygen atom becomes negative electrical charged (81) since oxygen atom (76), now, has a total of ten negative charged electrons (79a xxx 79n) in its "K" plus "L" orbits while maintaining it's original eight positive charged protons which makes up oxygen nucleus (83).
Since the hydrogen proton (84) (hydrogen nucleus) remains (after covalent link up), then the hydrogen atom takes-on a positive charge (78) co-equalling the positive charge of the hydrogen nucleus proton (84).
Together, the total net charge of water molecule (85) is zero despite the fact that each water molecule atom retains
its electrical charge.
In other words, water molecule (85) is a electrically bipolar molecule having a stable configuration of charged atoms bound together by electrostatic force (qq').
Electromagnetic bonding forces between unlike atoms (76n7) are negligible or non-existence, since oxygen atom (76) electrons are paired together, while rotating in opposite direction which, in turn, causes oxygen atom (76) to be electromagnetically neutral to hydrogen atom (77).
Electron theory of magnetism requires orbital electrons to spin in the same direction before an atom can exhibit a electromagnetic field.
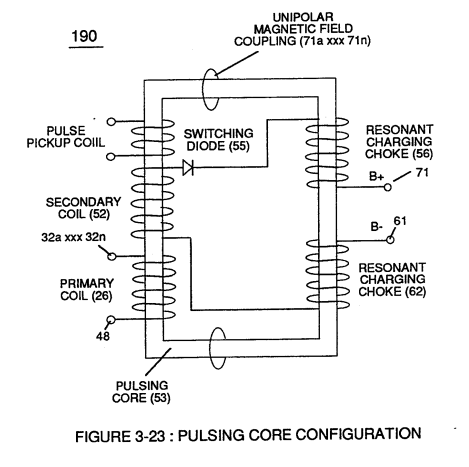
Furthermore, external electrical force (66/67) can alter the electromagnetic properties of a atom since electromagnetic force is dependent on the movement of charged particles in a electrostatic field voltage Intensifier circuit (190) of figure (3-23), now, allows voltage to dissociates water molecule (85) by overcoming electrostatic bonding force (qq') between unlike atoms (76n7) while restricting amp flow, as illustrated in (160) of Figure (3-26).
|
Figure (3-23) |
Figure (3-26) |